Substance
Delay discounting
Go/no-go
SSRTT
5-CSRTT
Cocaine
Acute
?
⇑ (66)
?
⇑ (67)
rep/chronic/self-admin
⇔ (61)
⇓ (69)
⇔ (70)
Amphetamine
Acute
⇑ (72)
⇑ (73)
rep/chronic/self-admin
⇔ (75)
?
?
⇔ (76)
Heroin/morphine
Acute
?
⇔ (79)
⇑ (79)
rep/chronic/self-admin
?
?
?
⇔ (70)
Alcohol
Acute
⇑ (80)
?
⇑ (73)
⇑ (81)
rep/chronic/self-admin
?
?
?
?
Alc. pref. bred animals
⇑ (84)
?
?
Nicotine
Acute
?
?
⇑ (A. Bari and T.W. Robbins, unpublished findings)
rep/chronic/self-admin
⇔ (86)
?
?
⇑ (87)
Therapeutic drugs
Methylphenidate
⇓ (71)
?
⇓ (88)
Atomoxetine
⇓ (90)
?
⇓ (90)
⇓ (89)
Modafinil
?
?
⇓ (88)
⇑ (91)
SSRIs
?
⇔ (93)
?
Benzodiazepines
⇑ (95)
⇑ (A. Bari and T.W. Robbins, unpublished findings)
⇑ (81)
3.2 Stop Signal Reaction Time and Go/No-Go Tasks
The most widespread tests of response inhibition are the go/no-go and SSRT paradigms. These tasks have been successfully adapted for use in animals and have high face and predictive validity. In the go/no-go paradigm, subjects are required to respond to a cue – the go signal – and not to respond to a different infrequent stimulus – the no-go signal. Impaired go/no-go performance has been reported in children with ADHD (105) and in individuals using different classes of drugs, including cocaine (106, 107), alcohol (108), tobacco (109), but not MDMA (the principal component of ecstasy) or cannabis (110). In animals, acute (66), but not chronic (61) administration of cocaine impairs behavioural inhibition on the go/no-go task.
The SSRT task is a sophisticated variant of the go/no-go task where subjects are required to cancel an already initiated motor response following the presentation of an unexpected stop-signal (usually auditory or visual) (25). The action to be inhibited is made prepotent by its high frequency and fast execution. Stop trials (usually 20–25% of total trials) are randomly interspersed among go trials in order to make the stop-signal unpredictable for the subject. By varying the timing of the stop-signal, it is possible to measure the duration of the inhibitory process itself (the SSRT) and to derive an inhibition function (25, 40). Previous research has demonstrated that the SSRT is consistently longer and more variable in ADHD individuals (111, 112) as well as in drug abusers (113–115).
The SSRT is decreased in rats by drugs commonly used for the treatment of ADHD, although for some drugs – especially the psychostimulants – the effect is dependent on baseline performance (73, 88, 116). Stopping efficiency is also improved by non-stimulant drugs such as atomoxetine, an effect that has been observed in both rats and humans. However, unlike psychostimulant drugs, this effect does not appear to depend on baseline performance (90, 93, 117). The exact mechanism of atomoxetine’s beneficial action is still unclear but may involve increased noradrenaline (NA) function in the prefrontal cortex (PFC) since GBR-12909, a selective DA reuptake inhibitor, tends to speed go responses but not stop responses; whereas guanfacine, a selective alpha-2 adrenoceptor agonist, which diminishes ascending noradrenergic activity (118, 119), slows SSRT and impairs stop-signal accuracy in the rat (93). Moreover, the speeding effect of methylphenidate on the SSRT in slow-stopping animals is not blocked by the DA receptor antagonist cis-flupenthixol (88), suggesting a non-dopaminergic mechanism of action. Finally, in ADHD children, increasing dopaminergic transmission by l-dopa administration does not appear to influence SSRT, while the tricyclic antidepressant desipramine – which inhibits the reuptake of NA – decreases SSRT (120). Thus, noradrenergic neurotransmission appears to be important for the inhibition of an already initiated response (41), whereas DA appears to selectively modulate the go response, potentially at the level of the striatum (121, 122). Conversely, 5-HT seems to have a marginal role, if any, in this form of behavioural inhibition because 5-HT global depletion, acute tryptophan depletion and citalopram administration do not alter SSRT in humans and rats (93, 117, 123, 124).
3.3 Five-Choice Serial Reaction Time Task (5-CSRTT)
The 5-CSRTT is an automated operant behavioural task used widely for the assessment of sustained attention and impulsivity in rodents (125, 126). The basic task is modelled on the continuous performance task used to study human attentional processes (127, 128). The rodent version of the task requires animals to detect brief flashes of light presented pseudo-randomly in one of five holes and to make a nose-poke response in the correct spatial location in order to receive a food reward. Rats are trained to monitor a horizontal array of apertures and to withhold from responding for a fixed or variable inter-trial interval (ITI) until the onset of the stimulus (see (129) for details). Generally, the accuracy of stimulus discrimination provides an index of attentional capacity, while premature responses – made before the presentation of the stimulus – are regarded as a form of impulsive behaviour and hence a failure in impulse control (125).
Acutely administered stimulant drugs invariably increase impulsive behaviour on the 5-CSRTT (125), an effect likely mediated by increased DA activity in the nucleus accumbens (NAC) (130, 131). On the other hand, atomoxetine, which produces no appreciable effects on subcortical DA (132), decreases impulsivity on the 5-CSRTT (89, 90, 133). The selective depletion of brain 5-HT by intracerebroventricular infusions of 5,7-dihydroxytryptamine (5,7-DHT) in adult rats produces long-lasting hyperactivity as well as increased impulsivity on this task (134). Attentional impairments on the 5-CSRTT mainly result from selective lesions of the cortical cholinergic system and, under certain conditions, following disruption of the ascending noradrenergic system (126, 135–139).
Recent research has highlighted a strong link between impulsivity on the 5-CSRTT and different stages of the drug addiction cycle. Thus, animals selected for high levels of premature responses (high impulsive rats; HI) display higher rates of cocaine and nicotine self-administration compared with low-impulsive rats (LI) (52, 140). Moreover, as we will discuss in the next section, high impulsivity on the 5-CSRTT predicts the transition to compulsive drug seeking and taking (14).
4 Role of Impulsivity in the Drug Addiction Cycle
Scientific research on drug addiction has focused traditionally on motivational processes that lead to, and are altered by, pathological drug use. For example, repeated drug use has been hypothesised to increase the incentive motivational properties of drugs and drug cues and stimuli associated with drug use (141). This effect may bias decision-making processes toward drug procurement and the immediate euphoria-producing effects of drugs at the expense of future negative outcomes. Indeed, chronic exposure to stimulant drugs is associated with morphological abnormalities in a number of brain areas involved in behavioural inhibition, impairing the ability of the subject to refrain from using drugs and discounting negative future consequences of chronic drug abuse (6, 31).
More recently, an important role for impulsivity and other behavioural traits in drug addiction has been recognised (8, 31, 142). It has been found, for example, that subjects displaying high levels of exploratory behaviour and sensation/novelty-seeking are more likely to initiate drug use (24, 143, 144). Thus, animals showing increased novelty-induced locomotor activity (i.e., the ‘high responder rat or HR’) show a greater propensity to self-administer psychomotor stimulant drugs such as amphetamine and cocaine (143, 145), a characteristic postulated to be mediated by increased activity of the brain dopaminergic systems (146).
Other studies have shown that individuals with personality traits related to impulsivity begin drug use earlier and show a generally higher rate of drug abuse than the general population (147–151). However, the precise link between impulsivity and the initiation of drug use is still a matter of considerable debate and uncertainty. It is possible that people start to use drugs because they value the immediate rewarding or pleasurable effects of the drug more than future larger goals, such as stable personal relationships and a rewarding career. Such individuals tend to be insensitive to delayed gratification (55, 56, 152). Other forms of impulsivity – such as impaired inhibition in the stop task – also correlate with drug use (113–115), an association mediated in part by the failure of such individuals to inhibit behaviour directed towards drug-associated environmental cues (31). An alternative explanation is that individuals with personality traits related to extroversion (153) tend to be exposed to more diverse environments compared with introverted individuals, thus increasing their probability of coming into contact with other drug users (154). Once the drug is available in the immediately surrounding environment, factors such as peer pressure, underlying impairments in behavioural inhibition, disregard for future negative consequences, reward dependence and low harm avoidance all hypothetically increase the probability of experimentation with drugs. Accordingly, de Wit and Richards (31) describe the addiction process as being determined by heightened reward sensitivity and decreased behavioural inhibition. These characteristics are thought to combine together to increase the likelihood of initiating substance use and relapse after abstinence. According to Dawe and colleagues (155), individuals with high reward sensitivity would experience greater pleasure by using the substance, while poor behavioural inhibition would result in the continuation of drug use despite negative consequences. A third causal pathway in the development of drug addiction relates to ‘stress reduction’ (156, 157), consistent with the ‘self-medication’ hypothesis of addiction (158). Thus, poor behavioural inhibition and the excessive pursuit of rewarding activities that are often socially unacceptable and/or illegal can lead to undesirable consequences (e.g., social isolation, loss of employment), which may drive drug use as a means to alleviate the distress caused by such events.
A number of studies in laboratory animals have supported a causal link between impulsivity and drug addiction, specifically by influencing the different stages of the drug addiction cycle, (e.g., (8)). For example, underlying deficits in delay discounting have been shown to influence the acquisition or initiation of both cocaine (51, 53) and ethanol self-administration (54). The maintenance phase of drug use, which is often accompanied by drug bingeing and escalation (159), is influenced by spontaneously high levels of impulsivity on the 5-CSRTT. Thus, rats selected for high impulsivity on the 5-CSRTT show a robust escalation of cocaine (140) and nicotine (52) self-administration. Therefore, ‘trait-like’ impulsivity on the 5-CSRTT appears to predict the escalation, but not the initiation of drug intake, which instead is predicted by a high locomotor response to novelty (i.e., the HR phenotype).
High impulsivity on the 5-CSRTT also predicts the transition or switch to compulsive drug seeking as shown using a rat model based on three key diagnostic criteria of drug abuse from the DSM-IV, namely, increased motivation to take the drug (criterion 6), an inability to inhibit drug seeking (criterion 3), and continued drug use despite negative or adverse consequences (criterion 7) (14). The degree of impulsivity, as measured by the 5-CSRTT, was found to correlate with the propensity of the rats to compulsively self-administer cocaine as measured by the resistance of HI rats to punishment-induced suppression of responding for cocaine. Thus, ‘trait’ impulsivity appears to play a causal role in facilitating the transition from initial drug exposure to habitual and ultimately compulsive form of drug taking (see Fig. 1).
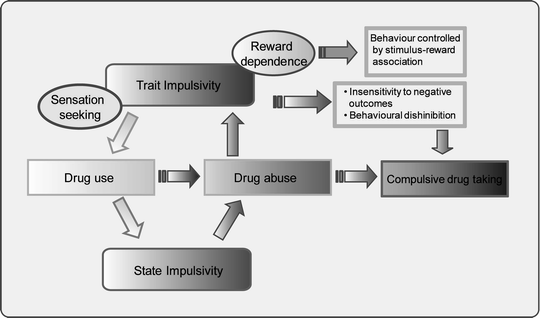

Fig. 1.
Hypothetical inter-relationship between impulsivity and substance abuse. Pre-existing impulsive traits are postulated to facilitate drug use (light grey pathway) in part by short and long-lasting interactive effects of chronic drug exposure on behavioural inhibition and reward sensitivity (dark grey pathway). Trait impulsivity in this schema includes both clinical forms – caused by psychiatric disorders such as ADHD or acquired brain damage – and non-clinical, stable personality subtypes. State impulsivity is hypothesised to be triggered by initial drug use, stressful life events and, in the more advanced stage of drug use, by drug-related stimuli leading to craving and chronic relapse to drug taking activities. Both trait and state forms of impulsivity are assumed to lead to maladaptive decision-making which, in turn, encourage further drug use to alleviate the distress caused by increasingly negative life events.
Finally, there is recent evidence that ‘trait’ impulsivity in rats can influence the propensity for relapse to drug seeking and taking under certain conditions (160, 161). Thus, drugs that reduce impulsivity (e.g., atomoxetine) may also lessen the likelihood for relapse or reinstatement of drug taking after a period of abstinence.
5 Neuroanatomical Substrates
The high co-morbidity between clinical impulsivity and substance use/dependence is postulated to reflect an overlapping pattern of neural and neurochemical abnormalities in limbic fronto-striatal brain networks leading to a characteristic impairment in cognitive control over behaviour (6, 162). Such impairments in control may be determined in part by genetic influences in the case of antecedent or trait impulsivity (1), or via harmful interactive effects on PFC functioning of chronic exposure to stimulant and opiate drugs (see Fig. 1). In the latter case, chronic exposure to drugs is hypothesised to result in a form of cognitive impulsivity (or ‘state’ impulsivity) produced by (i) frontal cortical dysfunction leading to inhibitory control deficits and (ii) an augmentation of the incentive motivational properties of stimuli associated with drug use, putatively via impaired subcortical processing of stimuli at the level of the amygdala and NAC (6).
In the schema shown in Fig. 1, both ‘trait’ and ‘state’ forms of impulsivity are hypothesised to facilitate the transition from first drug use to repeated use and addiction by increasing the control over behaviour by stimulus–reward associations and by decreasing fronto-cortical functioning. The net result is a failure to adequately evaluate the consequences of risky or inappropriate drug taking behaviour, thereby facilitating continued drug use leading in turn to a further exacerbation and impairment in inhibitory control mechanisms mediated by the PFC and related structures (see Fig. 2).
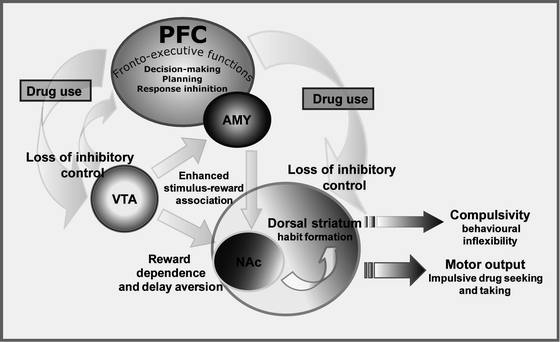

Fig. 2.
Simplified schema showing how chronic drug exposure progressively impairs the inhibitory influence of the PFC and evokes a shift in dopamine function from ventral (i.e., NAC) to more dorsal areas of the striatum, especially those domains implicated in habitual forms of behaviour. In brief, chronic drug abuse is believed to impair the inhibitory influence of the PFC on striatal and mesostriatal structures (light grey curved arrows) resulting in behaviour that is progressively controlled by immediate, reward-related impulses. Hyperactivity of the mesolimbic dopaminergic system is thought to enhance stimulus-reward associations (6) (light grey straight arrows) leading to habitual drug taking − mediated by more dorsal regions of the striatum (163) − as well as increased susceptibility for relapse even after long periods of drug abstinence.
The core pathologies of ADHD and drug addiction are hypothesised to involve frontal cortical brain regions (dorsolateral PFC, anterior cingulate cortex, orbitofrontal cortex OFC) and basal ganglia structures, including especially the NAC and caudate nucleus (collectively, the striatum). In human addicts, the presentation of drug-related stimuli produces a reliable activation of limbic brain structures (164, 165) whilst cocaine abusers exhibit marked morphological abnormalities in OFC (166) and are impaired on tasks dependent on OFC function such as probabilistic reversal learning and gambling tasks (167, 168). They are also impaired on go/no-go and stop task paradigms (106, 107, 114). Several studies confirm these findings with evidence of structural and metabolic abnormalities in the PFC and striatum following extended drug exposure both in humans and other animals (e.g., (101, 169)). Indeed, our own research has revealed lasting impairments in inhibitory response control, attention, and motivational variables in rats exposed contingently to intravenous methamphetamine, MDMA and heroin (70, 76, 170), consistent with recent reports showing enduring effects of cocaine on working memory and sustained attention in rats (171, 172), as well as tasks sensitive to OFC function (173–175).
The main neural loci mediating different forms of impulsivity in rodents, including delay discounting impulsivity, SSRT and impulsive responding on the 5-CSRTT, have been extensively investigated in recent years (see (8, 16, 176, 177) for recent reviews on this topic). Key findings include the demonstrations that impulsive choice on the DD paradigm is increased by selective lesions of the core, but not shell sub-region of the NAC (178, 179) and by lesions of the OFC ((180, 181), but see (182)). Other forms of impulsivity, including impulsive actions, depend on the functional integrity of the medial PFC, especially the anterior cingulate cortex and infralimbic cortex (183) as well as the NAC and areas of the medial striatum considered homologous to the caudate in humans (116, 176, 184). Taken together these findings suggest a level of convergence in the neural systems that underlie distinct forms of impulsivity that may reflect more general psychological processes such as ‘waiting’ or ‘stopping’ impulsivity (123). Indeed, rats selected for spontaneously high levels of impulsivity on the 5-CSRTT also show delay intolerance on the DD paradigm but are unimpaired on other forms of impulsivity, including the SSRT task and Pavlovian conditioned approach (185).
Previously, we reported using dedicated small animal positron emission tomography (PET) that hyper-impulsive rats on the 5-CSRTT have a reduced availability of dopamine D2/3 receptors in the ventral striatum (140). They also maintain significantly higher rates of intravenous cocaine and nicotine self-administration (52, 140). The magnitude of the change in dopamine D2/3 receptors was inversely related to the severity of impulsivity suggestive of a possible causal link between dopamine D2/3 receptors and impulsivity, consistent with previous findings of delay aversion in rats with selective lesions of the NAC (178). These and other data (51) indicate that impulsivity – as defined by the inability to wait or bridge delays to future reinforcement – may be causally involved in drug abuse vulnerability rather than the other way around. Thus, the reduced availability of dopamine D2/3 receptors in abstinent cocaine addicts (186) may in part be a pre-existing abnormality that confers risk for drug bingeing which in turn accelerates the transition from controlled drug use to habitual and ultimately compulsive patterns of drug taking (161).
6 Concluding Remarks
In this chapter, we have highlighted recent significant advances in our understanding of the role of impulsivity in the drug addiction cycle together with putative neural substrates. However, it is clear that much further work is needed to elucidate (i) the precise modifications caused by drugs of abuse on neuronal circuits involved in behavioural inhibition and reward sensitivity and (ii) the causal role and brain mechanisms of impulsivity in substance use disorders. Animal models provide a valuable source of convergent information because they allow drug exposure to be precisely controlled whilst, in addition, providing a metric of cognitive and behavioural performance prior to drug experience. Studies in human addicts are often confounded by interpretative issues relating to variable drug histories and pre-morbid intellectual capabilities.
In closing, it is worth noting that many of the drugs used to calm hyperactive and impulsive children are themselves widely abused by humans (187). A major challenge for future research will be to determine whether prescribed stimulant treatment of ADHD (e.g., with Ritalin®) offers protection against future problem drug use or instead exacerbates and speeds up the transition to compulsive drug use in susceptible individuals. It will also be important to compare these effects with newer non-stimulant drugs such as atomoxetine, which have proven clinical efficacy in ADHD (188, 189). Finally, a major objective for future research should be to continue to develop sophisticated animal models with high construct and face validity to investigate further the neural and psychological mechanisms underlying the switch from impulsivity to compulsive drug seeking and taking and how these changes interact with vulnerable personality traits and chronic drug exposure.
Acknowledgements
The work reviewed in this chapter was funded by grants from the MRC (G0401068, G0600196, G0701500, G0802729), the Wellcome Trust (076274/z/04/z) and by a consortium joint award from the MRC and Wellcome Trust (G0001354) within the Cambridge University Behavioural and Clinical Neuroscience Institute. AB was supported by a studentship from the MRC.
References
1.
Kreek MJ, Nielsen DA, Butelman ER, LaForge KS (2005) Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci 8(11):1450–1457PubMed
2.
Verdejo-Garcia A, Lawrence AJ, Clark L (2008) Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev 32(4):777–810PubMed
3.
Sarramon C, Verdoux H, Schmitt L, Bourgeois M (1999) Addiction and personality traits: sensation seeking, anhedonia, impulsivity. L’Encephale 25(6):569–575PubMed
4.
Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC (2001) Psychiatric aspects of impulsivity. Am J Psychiatry 158(11):1783–1793PubMed
6.
Jentsch JD, Taylor JR (1999) Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology 146(4):373–390PubMed
7.
Volkow ND, Fowler JS, Wang GJ (2002) Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behav Pharmacol 13(5–6):355–366PubMed
8.
Perry JL, Carroll ME (2008) The role of impulsive behavior in drug abuse. Psychopharmacology 200(1):1–26PubMed
9.
Olmstead MC (2006) Animal models of drug addiction: where do we go from here? Q J Exp Psychol 59(4):625–653
10.
Rogers RD, Robbins TW (2001) Investigating the neurocognitive deficits associated with chronic drug misuse. Curr Opin Neurobiol 11(2):250–257PubMed
11.
Koob GF, Le Moal M (1997) Drug abuse: hedonic homeostatic dysregulation. Science (New York, NY) 278(5335):52–58
12.
Koob GF, Le Moal M (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24(2):97–129PubMed
13.
Koob GF (2008) Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology 56(Suppl 1):18–31PubMed
14.
Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ (2008) High impulsivity predicts the switch to compulsive cocaine-taking. Science (New York, NY) 320(5881):1352–1355
15.
Milich R, Kramer J (1984) Reflections on impulsivity: an empirical investigation of impulsivity as a construct. Adv Learn Behav Disabil 3:57–94
16.
Winstanley CA, Eagle DM, Robbins TW (2006) Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev 26(4):379–395PubMed
17.
Patton JH, Stanford MS, Barratt ES (1995) Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51(6):768–774PubMed
18.
Barkley RA (1997) Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 121(1):65–94PubMed
20.
Eysenck SB, Eysenck HJ (1977) The place of impulsiveness in a dimensional system of personality description. Br J Soc Clin Psychol 16(1):57–68PubMed
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


