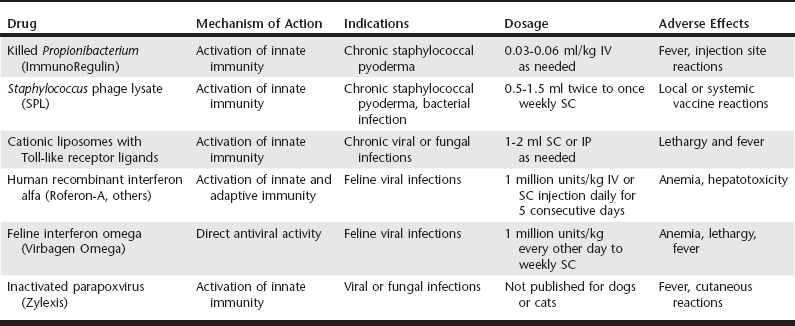
Chapter 262 At the other extreme, there are some infections in which the immune response to infection elicits such a marked inflammatory response that inflammation actually causes more injury to the host than the pathogen itself. Examples of these infections include acute rickettsial infections (e.g., E. canis and Rickettsia rickettsii infection), hemoplasma infections (e.g., Mycoplasma haemofelis, Candidatus Mycoplasma haemominutum), and some bacterial pneumonias and meningitides. For these infections, brief immunosuppression with glucocorticoids can reduce host inflammatory responses and thereby ameliorate the damage caused by unchecked inflammation, while at the same time not interfering with the overall effectiveness of treatment with conventional antimicrobial drugs (see Chapter 278). Type I IFNs, which actually consist of a family of closely related isoforms of IFN-α plus a single isoform of IFN-β, play a key role in early immune responses to viral infections and infections with certain bacterial pathogens. IFN-α is produced by all nucleated cells in the body in response to viral entry and replication and represents one of the primary immune defenses against viral pathogens (Seo and Hahm, 2010). For example, IFN-α suppresses viral replication in the host cell and augments cellular immunity, especially natural killer (NK) cell activity and CD8 T-cell responses. After chronic infection has become established, type I IFNs remain important, but IFN-γ responses become much more important as mediators of effector T-cell antiviral activity, including both CD4 T-cell and CD8 T-cell activity. IFN-γ plays a key role in regulating both early and late immunity to bacterial and fungal pathogens, and the importance of IFN-γ has been established clearly in a number of different animal models (Seder et al, 1999). Unlike the production of type I IFNs, the production of IFN-γ is restricted to only a few cell types in the body, primarily NK cells and T lymphocytes. Early after infection, IFN-γ is produced primarily by NK cells, whereas later in the course of infection, IFN-γ production is restricted to antigen-specific CD4+ and CD8+ T cells. Production of IFN-γ by T lymphocytes is largely responsible for maintaining sustained immune control of bacteria, fungal, and viral infections. IFN-γ has numerous immune effects, including direct suppression of intracellular replication as well as induction of effective intracellular killing mechanisms by macrophages and neutrophils. Both type I and type II IFNs are important for controlling persistent infections by viruses, fungi, and bacteria. Therefore the most effective immunotherapeutics are those that produce balanced induction of both type I and type II IFN responses. For example, Zylexis, a veterinary immune stimulant produced from inactivated parapoxviral particles, has been shown potently to induce IFN production (Fachinger et al, 2000). Type I IFN and type II IFNs exert critical and nonredundant activities against intracellular pathogens. However, although most currently licensed veterinary immune therapeutics can elicit some IFN-γ production, few are strong inducers of IFN-α production, with the possible exception of Zylexis. Thus there is still a need for veterinary immune therapeutics capable of potently activating both IFN-α and IFN-γ production in vivo. Effective immunotherapeutics should induce sustained immune activation and IFN production, which may require the administration of relatively long-acting immune stimulants (e.g., live bacille Calmette-Guérin) or repeated administration of shorter-acting immune activators (e.g., Toll-like receptor 3 [TLR-3] or TLR-9 agonists; see Table 262-1). For example, effective intracellular killing of persistent intracellular pathogens often requires prolonged exposure to activating concentrations of IFNs because effective pathogen elimination typically takes time to develop. This principle is one of the primary drawbacks to the use of recombinant cytokines, such as human recombinant IFN-α (Roferon-A, others), for treatment of persistent viral infections in cats. Frequent and repeated dosing also means that there is a higher likelihood of development of neutralizing antibodies against human recombinant cytokines such as IFN-α (Zeidner et al, 1990). The use of recombinant feline IFN omega (currently available only in Europe) avoids the issue of neutralizing antibodies, but long-term, frequent treatment still is required, and optimal daily dosing regimens have not been established for most pathogens. Currently little published information is available allowing direct comparison of the ability of commercially available immune stimulants to induce production of cytokines (and especially IFN-γ). However, several general trends can be discerned from experimental studies of different classes of immune therapeutics. Immune therapeutics based on nucleic acids (or inactivated viral particles) appear to be the most effective inducers of both type I and type II IFN responses. For example, Zylexis was shown to induce IFN-γ production by porcine peripheral blood mononuclear cells, most likely by activation of the TLR-3 pathway (Fachinger et al, 2000). Likewise, an immune stimulant comprised of cationic liposomes and noncoding plasmid DNA (a TLR-9 agonist) has been shown by us and others to induce high levels of IFN-α and IFN-γ production in mice, cats, and dogs (Kamstock et al, 2006; Veir et al, 2006). In contrast, immune stimulants based on purified or enriched bacterial or fungal extracts tend to induce high levels of tumor necrosis factor-α (TNF-α) production, but lower amounts of IFN-γ and little or no IFN-α production. For example, killed Propionibacterium acnes (ImmunoRegulin) has been reported to induce TNF-α production by bovine leukocytes. Likewise, Staphylococcus phage lysate (SPL) has been reported to activate innate immunity. Cell wall extracts of Aloe vera (Acemannan Immunostimulant), which are rich in polysaccharides, are reported to induce TNF-α production by macrophages in vitro (Zhang and Tizard, 1996).
Immunotherapy for Infectious Diseases
Role of Immune-Stimulatory and Immunosuppressive Therapy
Principles of Immune-Stimulatory Therapy
Key Role of Type I Interferons in Antiviral Immunity
Major Role of Interferon-γ in Antibacterial and Antifungal Immunity
Effective Immune Therapeutics and Balanced Interferon Production
Importance of Sustained Interferon Production
Cytokine Induction Profiles of Currently Available Immune Therapeutics
Immunotherapy for Infectious Diseases
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree