Fig. 50.1
Immunolocalization of serum protein s in the tumor tissues formed by the Lu65 cells and prepared with cryobiopsy (Cb). Their serial sections were stained with hematoxylin-eosin (HE, a) or immunostained for albumin (b), immunoglobulin G 1 (IgG1 ; c), and IgM (d). b and c. Both albumin and IgG1 are clearly immunolocalized in the tumor blood vessel s (arrowheads) and also in the interstitial matrix between the tumor cells (small arrows). d. On the other hand, IgM immunostaining is detected mainly in the tumor blood vessels (arrowheads) and the connective tissue of the capsule around the tumor mass. Other serum proteins, albumin and IgG1, are also observed in the connective tissue around the tumor mass (a–d, large arrows). Bars a–d, 50 μm; inset, 10 μm
50.3 Immunolocalization of Intrinsic Serum Proteins in Xenografted Lung Larger Cell Carcinoma Tissue with FQF
Conventional immunohistochemical techniques have some problems of molecular diffusion artifact and antigen masking for the detection of immunoglobulin in animal organs, which were already reported in large malignant cells like the Reed-Sternberg cells of Hodgkin’s lymphoma [18]. In the present study, the diffusion artifacts of both albumin and IgG1 were also observed in tumor cells prepared by FQF (Fig. 50.2). However, such technical artifact s can be mostly prevented by IVCT and Cb, because cells and tissues in target organs of living animal bodies are immediately cryofixed at the time of freezing in situ.
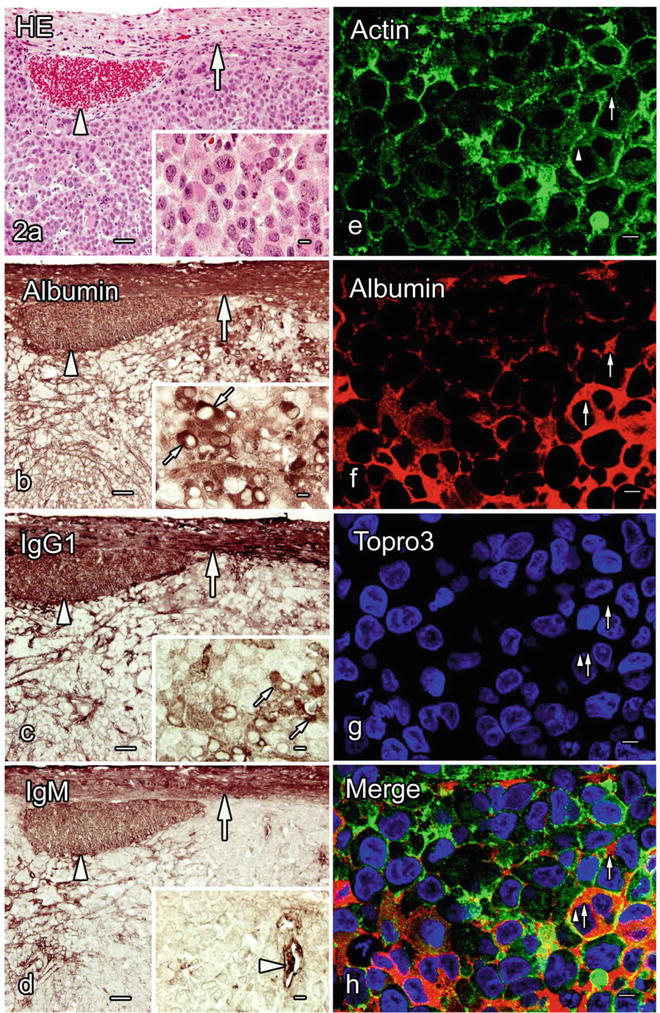

Fig. 50.2
Immunolocalization of serum protein s in the tumor tissues formed by Lu65 cells, which were prepared with the quick-freezing of fresh resected tissues (FQF). a–d. Light micrographs of the serial paraffin sections stained with hematoxylin-eosin (HE) or immunostained for albumin (b), immunoglobulin G 1 (IgG1 ; c), and IgM (d). Both albumin and IgG1 are immunolocalized in the tumor blood vessel s (b, c, arrowheads) and the interstitium (b, c, large arrows), as well as in the cytoplasm of some tumor cells (b, c, small arrows in insets). IgM immunoreactivity is also detected in the interstitium (d, arrow) in addition to the blood vessels (d, arrowheads). e–h. Actin immunofluorescence is observed in the cytoplasm and along the cell membrane of the Lu65 cells (e, h, arrowheads). Albumin is immunolocalized in the tumor interstitium and the cytoplasm of some tumor cells (f, h, small arrows). All the serum proteins are also observed in the connective tissues around the tumor mass (a–d, curved arrows). Bars a–d, 50 μm (insets, 10 μm); e–h, 20 μm
50.4 Immunodistribution of Extrinsic BSA in Xenografted Lung Larger Cell Carcinoma Tissue with IVCT
The time-dependent immunolocalization of injected BSA was revealed within the tumor tissues, as shown in Fig. 50.3. The injected BSA was mostly immunolocalized within blood vessel s in the tumor mass and the capsular connective tissue 10 min after the injection, but later it was diffusely immunolocalized in the surrounding connective tissue and a fraction of the interstitium in the tumor mass. Some previous studies reported that BSA leaked out into the interstitial spaces within 1 h, using live imaging with intravital or a confocal laser scanning microscope [8, 19]. Small amounts of molecules may be difficult to visualize on histological sections by immunohistochemistry . However, because different intensities of immunoreactivity in a single histological section usually depend on the relative amount of active antigen sites, our findings suggest that the permeability of blood vessels in the tumor tissue is distinct from that in the surrounding connective tissue of the tumor capsule, which would partly depend on the molecular structure of blood vessels [16, 20]. In fact, we have detected abundant immunolocalization of BSA in the connective tissue of the tumor capsules 24 h after the BSA injection. In addition, 72 h after the BSA injection, BSA immunolocalization was more clearly detected in the interstitium of the tumor tissue. The repeated injection of BSA induced the accumulation of BSA in the blood circulation , thus causing the prolonged exposure of the tumor mass to higher concentrations of BSA over a few days, resulting in the increased concentration of leaked BSA in the tumor interstitium.
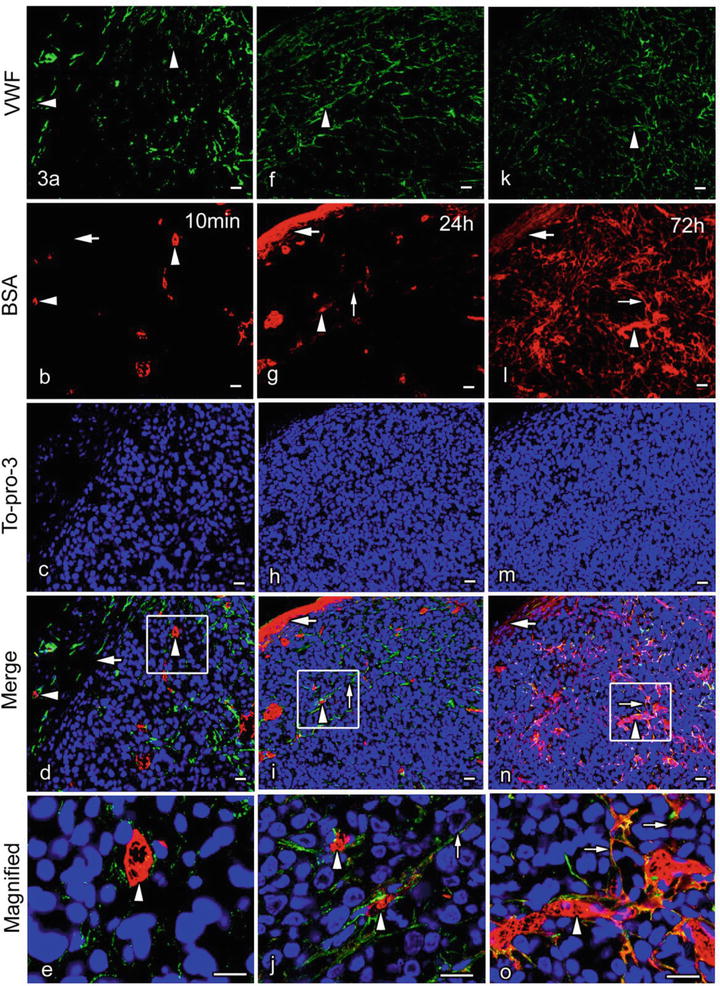

Fig. 50.3




Double-immunofluorescence micrographs of von Willebrand factor (vWF; green color) and intravenously injected bovine serum albumin (BSA; red color) 10 min (a–e) and 24 h (f–j) after the injection or at 72 h of the daily BSA injections (k–o), which were obtained with cryosections of the tumor tissues formed by the Lu99 cells and prepared with IVCT. a–e. BSA immunostaining can be mostly observed in some blood vessel s immunopositive for vWF (a, b, d, e, arrowheads) 10 min after the BSA injection, although it is not detected in the surrounding connectivity tissue of the tumor capsule (b, d, large arrows). f–j. However, 24 h after one shot of BSA injection, BSA immunoreactivity is slightly detected in the tumor interstitium (g, i, j, small arrows) as well as the tumor blood vessels (f, g, i, j, arrowheads). It is also strongly detected in the surrounding connective tissue of the tumor capsule (g, i, large arrows). k–o. BSA immunoreactivity is widely seen in the tumor interstitium (l, n, o, small arrows) outside the blood vessels (k, l, n, o, arrowheads) and also in the surrounding connective tissue (l, n, large arrows) because of daily injections of BSA at 72 h Bars 20 μm
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


