Fig. 23.1
Schematic drawing of experimental design and “in vivo cryotechnique ” (IVCT) used in the study [9]. Following two consecutive daily intraperitoneal injection of bovine serum albumin (BSA), kidney specimens of those mice were prepared with IVCT (a). Control specimens were obtained following intraperitoneal injection of half saline instead of BSA. To obtain kidney specimens with IVCT, left kidneys were gently exposed under anesthesia, and they were simultaneously cut with cryoknife and contacted isopentane -propane cryogen (−196 °C) using “in vivo cryoapparatu s ” (b). Rubber plates were placed under the kidney to prevent translocation upon cutting
IVCT yields fewer morphological artifacts through direct cryofixation of the living animal kidneys in vivo. During the subsequent freeze-substitution step at about −80 °C, organic solvent s with fixatives minimize diffusion of the serum protein s soluble to aqueous solution . Less compact cross-linking of structural components of cryotechniques preserves the antigenicity of molecules inside the tissue sections [14]. These advantages of IVCT presumably resulted in the clear immunolocalization of soluble serum protein s including BSA.
23.3 Immunolocalization of Various Serum Proteins
Immunostaining for IgG1 heavy chain (IgG1) , kappa light chain (Kappa), and mouse albumin along with BSA on serial sections prepared with IVCT revealed distributions of serum protein s with different molecular weights in the identical nephrons of the BSA-overload mice [9]. Immunolocalization of Kappa showed free kappa light chains and all types of immunoglobulins containing kappa light chains, while immunolocalization of IgG1 indicated the localization of intact IgG1 molecules. BSA, mouse albumin , and Kappa but not IgG1 were immunohistochemically detected in Bowman’s glomerular space and the apical cytoplasm of epithelium in proximal convoluted tubule s in nine nephrons out of 21 (Fig. 23.2) [9]. In the other nephrons, all of Kappa, IgG1, BSA, and mouse albumin could be detected in the Bowman’s spaces and proximal convoluted tubules (Fig. 23.2). Although both albumin and Kappa could be detected in almost all nephrons of the BSA-injected mice, immunoreactivity of albumin and Kappa was observed in seven glomeruli/five urinary tubules and in five glomeruli/six urinary tubules out of 20 nephrons of control mice, respectively [9]. IgG1 was not detected in any glomeruli or urinary tubules of nephrons from the control mice [9].
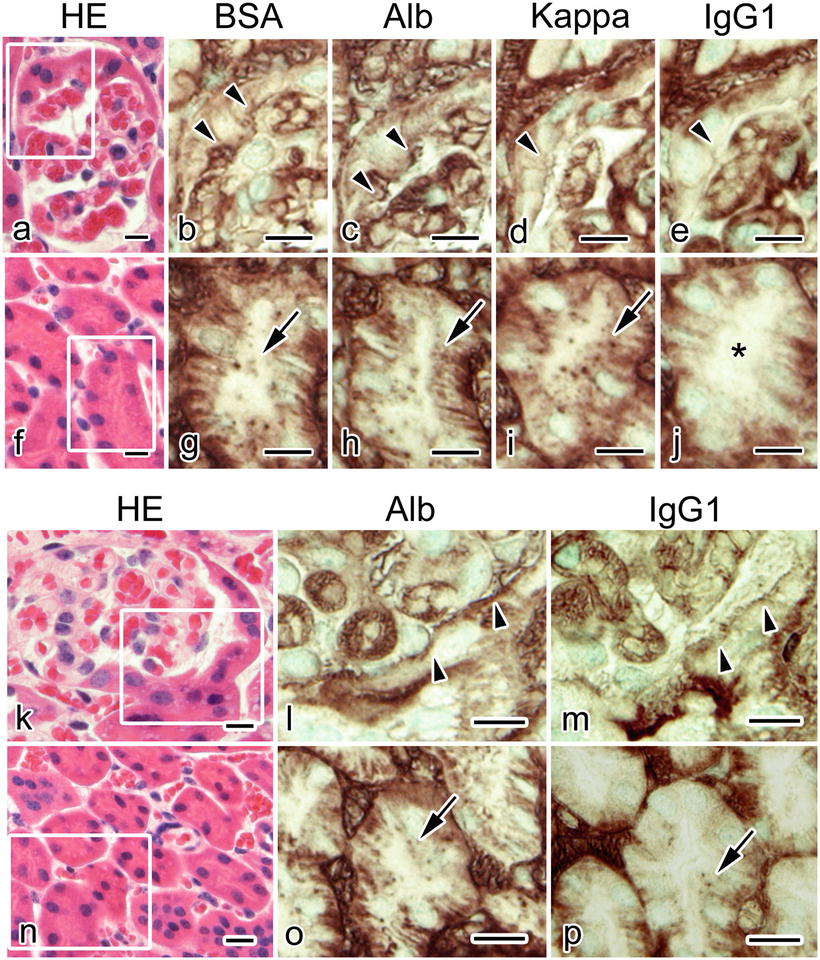

Fig. 23.2
Serial sections obtained from the BSA-overloaded mouse kidneys with “in vivo cryotechnique ” and stained with hematoxylin-eosin (HE; a, f, k, n) or immunostained for bovine serum albumin (BSA; b, g), mouse albumin (Alb; c, h, l, o), mouse immunoglobulin kappa light chain (Kappa; d, i), and mouse immunoglobulin G 1 heavy chain (IgG1 ; e, j, m, p) show that immunoreaction products of BSA, Alb, and Kappa, but not IgG1 (e, arrowhead; j, asterisk), are localized in Bowman’s space (b, c, d, arrowheads) and proximal convoluted tubule s (g, h, i, arrows) in some nephrons . In other nephrons, immunoreaction products of IgG1 are observed in the Bowman’s space (m, arrowheads) and proximal convoluted tubules (p, arrow), together with those of Alb (l, arrowheads; o, arrow). Serial sections of the areas marked with rectangles in (a), (f), (k), and (n) are immunostained and magnified in (b)–(e), (g)–(j), (l)–(m), and (o)–(p), respectively. Scale bars: 10 μm. (The figure was adapted from Zhou et al. [9].)
The immunolocalization of various molecular weight proteins which was different among nephrons in kidneys of the BSA-overload mouse might be attributable to defects in size and charge barrier of glomeruli [15]. Damage to the filtration barrier may first impair their charge-barrier functions and result in marked increase of albumin passage. The impaired charge barrier would first appear only in the limited number of nephrons, causing the functional heterogeneity of nephrons. Although the anionic sites of the glomerular basement membrane s were histochemically unaltered [10, 13], glomerular anions were lost in the similar rat model produced by BSA overload [16]. Severer damage on the barrier structures results in impairment of the size-barrier function, and HMW proteins, including IgG , pass through the barrier. Previous studies showed impaired size selectivity in the rat BSA-overload model by measurement of the clearances of neutral dextrans [15]. The impairment of glomerular size barrier was already present in some nephrons of living mice even after 2 days of the daily BSA injection. Since serum protein s with different molecular weights immunolocalized differently in nephrons, damages on many nephrons may be spatially and temporally variable in the BSA-overloaded mice.
23.4 Comparisons with the Conventional Preparation Methods
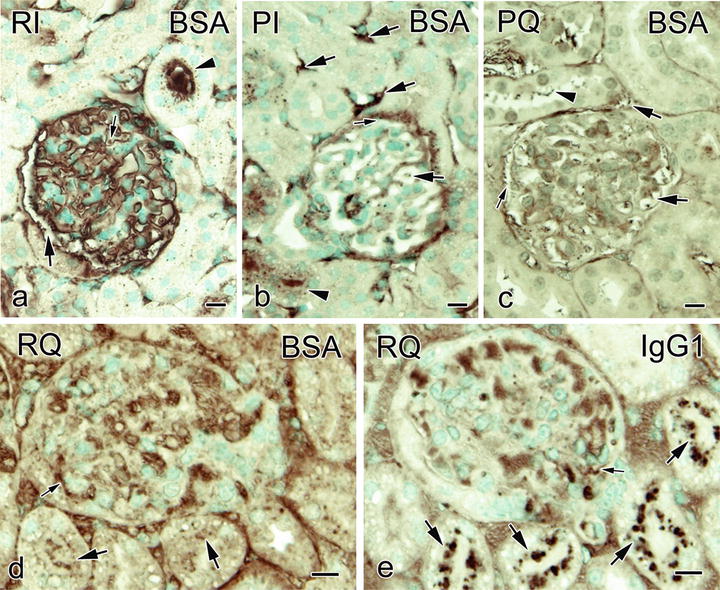
The immunostaining for BSA was weakly remained inside the collapsed glomerular capillary loop s , and more BSA immunoreactivity was observed in Bowman’s space with the conventional immersion-fixation followed by dehydration method (Fig. 23.3) [9]. The dot-like BSA immunostaining was also observed within some of the tubular lumens, but the immunoreactivity of BSA was little in the basal striation parts of urinary tubules (Fig. 23.3). With perfusion -fixation , open lumens of glomerular capillary loops were observed, and weak BSA immunostaining was found in proximal convoluted tubule s , Bowman’s space, and the glomerular or interstitial blood capillaries . With perfusion-fixation followed by immersion-fixation, the immunostaining patterns resembled tissue samples prepared by perfusion-fixation followed by quick-freezing (Fig. 23.3). With the tissue resection followed by quick-freezing, on the other hand, the immunostaining for BSA at basal striation portions in the urinary tubules was detected, and large immunoreaction products of IgG1 were also observed within Bowman’s space and in the apical cytoplasm of epithelial cell s in urinary tubules (Fig. 23.3). The findings clearly showed that the distribution of serum protein s was different among the preparation methods. The morphology as well as distribution of different molecules in kidneys can be affected by both ischemia and anoxia caused following common resection of tissue specimens. Heart arrest or tissue resection may cause translocation of the serum proteins into urinary space [17]. Furthermore, because of the artificial pressures which would wash out the serum proteins, perfusion-fixation significantly affects the immunolocalization of serum proteins [18–20].