Chapter 29
Immunocytochemistry
General Principles
The principle of ICC is the binding of antibody to antigen. Cells or tissues are incubated with antibodies developed to recognize a particular antigen. An understanding of antibody structure is important to fully comprehend and utilize this technique. The antibody is the key reagent for this process. The preferred isotype is immunoglobulin G (IgG) because of its more consistent generation and binding to antigen.1 Antibodies are “Y” shaped and are composed of constant and variable regions (Figure 29-1). The constant region of the Fc component has species-specific sites, which are unique to the animal species from which the antibody was generated.2 For instance, antibodies generated in a rabbit will bind to the constant region of rabbit antibodies. The variable region of the antibody recognizes specific epitopes of the antigen and is where antigen binding occurs. The N-terminal portion of the light chain and the variable region of the heavy chain are responsible for antigen binding.3 An antigen may consist of lipoproteins, glycoproteins, or peptides.1 The antibody consists of two heavy chains and two light chains, which form the constant and variable regions (see Figure 29-1). Light chains are identical to one another and may be one of two forms: kappa or lambda. Four different types of bonds contribute to the noncovalent antibody binding to antigen: (1) hydrophobic bonds, (2) hydrogen bonds, (3) ionic attraction, and (4) Van der Waals forces.2 The parameters and reagents used in ICC are aimed at enhancing the strength of binding. For example, Van der Waals forces are strengthened by increases in temperature, whereas electrostatic bonds may be enhanced by neutral pH, low ionic strength of buffer, and low incubation temperature.3
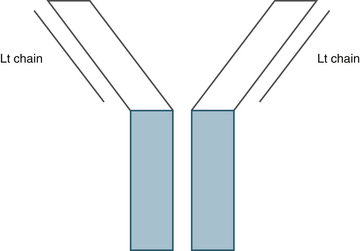
Figure 29-1 Antibody structure.
The blue areas indicate the constant region, and the gray lines represent the variable regions of the antibody.
Production of antibodies directed against a particular antigen is achieved by injecting purified antigen into a mouse or rabbit. Different types of antibodies are available for many types of antigens. Monoclonal antibodies recognize one particular epitope on an antigen and are, therefore, very specific. Polyclonal antibodies recognize multiple epitopes on a particular antigen. For this reason, they are less specific but more sensitive.2 Most of the antibodies produced and available for purchase are produced for use in human and murine tissues; therefore, in veterinary medicine, polyclonal antibodies are often used because of the increased sensitivity. Generally, monoclonal antibodies are produced in mice, and polyclonal antibodies are produced in rabbits, though monoclonal antibodies produced in rabbits are available for use in IHC and flow cytometry. Some proposed advantages for rabbit monoclonal antibodies include higher affinity of binding, larger volume of production, and production of antibodies against antigens that are not immunogenic in mice.4 This technology is still fairly new relative to mouse monoclonals, and the antibodies available are limited, so these antibodies may become more popular for ICC as more reagents become available.
Immunocytochemistry Technique
The primary antibody is directed against the cellular antigen of interest. The antibody may be directly conjugated to a labeling mechanism; or, more commonly, a secondary antibody conjugated to a labeling mechanism is used. This secondary antibody is directed against the species of production of the primary antibody. For example, a primary polyclonal antibody that is produced in a rabbit and binds CD79a, would use an anti-rabbit secondary antibody. This secondary antibody would bind the Fc component of the primary antibody produced in a rabbit (Figure 29-2, A). This technique has advantages and disadvantages. Use of a secondary antibody increases the intensity of staining because multiple secondary antibodies with labels attached may bind to a single primary antibody (see Figure 29-2, B). The main disadvantage is that a greater chance of background staining exists when using a secondary antibody because of an increased chance of nonspecific binding.2
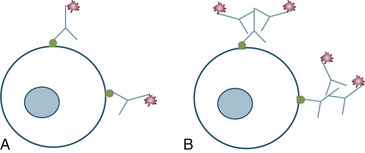
Figure 29-2 Antibody binding to cellular antigen.
A, Primary antibody with detection marker conjugated to the constant region of the antibody. B, Primary antibody recognized by species-specific secondary antibody with detection marker attached to the secondary antibody.
Depending on the system being used, the primary antibody, or more likely the secondary antibody, is attached to a labeling mechanism. Labels may consist of a fluorochrome, an enzyme or particulate matter (e.g., gold). Fluorescence was the first type of label used for immunocytochemistry back in 1942.5 The theory behind fluorescence is the fluorescent label absorbs one wavelength of light and then emits a higher wavelength of light. A microscope capable of producing a specific wavelength of light is necessary for this technique. The photons for emission and excitation are separated by optical filters.2 The staining fades over time, so this method is less ideal for use in a diagnostic laboratory where samples need to be saved for long periods.
Enzymatic labels are used much more commonly in ICC. With this technique an enzyme is attached to the antibody, and the sample is incubated with a substrate, which will interact with the enzyme. This interaction allows production of a colored reaction product.2 The reaction is permanent, so it is more appropriate for samples that need to be stored for several years.
Controls
When submitting slides for ICC, it is important to consider the controls necessary so that adequate numbers of slides are submitted. Positive and negative controls should be run for each antibody, and it is important that the controls match the sample type.6 For example, it is inappropriate to use paraffin-embedded, formalin-fixed tissues as a positive or negative control for air-dried cytology specimens not fixed in formalin. If the patient’s specimen has undergone formalin fixation with antigen retrieval, so should the control. The majority of antibodies available for purchase have undergone extensive validation ensuring that the antibody is specific for the antigen being analyzed. At a minimum, a Western blot analysis should be performed to show that the antibody recognizes the antigen of an appropriate molecular weight. Additionally, two different antibodies recognizing different epitopes of the same antigen may be used to show that the antibodies co-localize on the same antigen.5,7 These tests do not need to be done with each sample but should be done as part of the initial validation process of the antibody. A positive control that is more appropriate for diagnostic use is cells from a tissue known to contain cells that express the antigen of interest. Slides prepared from a lymph node could serve as a positive control for CD79a, CD20, CD3, CD4, and CD8.
The secondary antibody control is often used as a negative control. The purpose of this control is to show that the secondary antibody is binding to the primary antibody and not nonspecifically binding to unrelated structures. Several options exist, but commonly, the primary antibody is omitted on this slide and replaced with serum from the same species.2,5 This control should be performed on a slide containing cells from the patient.
Antigen Retrieval
One of the advantages of ICC is that it can be performed on air-dried slides that are not exposed to formalin. However, air-dried slides used for cytology are only good for ICC for several weeks; this is a major limitation of ICC, especially when trying to save positive control slides. As a solution to the temporary nature of cytology slides, 10% neutral buffered formalin can fix slides indefinitely.8 However, formalin results in cross-linking of deoxyribonucleic acid (DNA) and alters the structure of proteins, thereby limiting the ability of antibody recognition of a particular antigen.9 To address this problem, the process of antigen retrieval was identified in the early 1990s.10 Paraffin-embedded, formalin-fixed tissues are boiled to reverse the chemical reactions between formalin and protein.3 Antigen retrieval for cytologic specimens has been evaluated by Valli et al.8 Heating the sample in normal saline will work for cytologic specimens.8 The samples may be heated by using something as common as a microwave or as specialized as a decloaking chamber (Biocare Medical, Walnut Creek, CA). If the samples are processed and stained within 24 hours, the process of cell fixation and antigen retrieval is unnecessary for cytology, but for older samples, formalin fixation with antigen retrieval may provide excellent results (see Figure 29-3).
Sample Submission
Air-dried slides submitted for cytology are acceptable for use for ICC. If the sample is left at room temperature, the cell viability is limited to days. The viability of unstained slides may be prolonged by freezing the slides at −4°C. Slides should be placed in a plastic container and sealed in a zip lock bag containing dessicant.11 Alternatively, the slides may be dipped in 10% formalin; however, as mentioned above, antigen retrieval will need to be performed with these slides.8 Samples may also be submitted in transport media. Some laboratories that perform ICC will provide tubes containing transport media. The media should consist of saline with 25% to 30% protein, either bovine serum albumin or fetal calf serum (Box 29-1). Acetone may be added to the sample to limit bacterial growth; however, acetone permeabilizes the cell membrane and, therefore, may result in cell lysis.2 If the sample is processed quickly, the acetone will have minimal impact on the quality of the sample, but after 2 to 3 days, the cellular lysis is considerable (Figure 29-4). For microscopic evaluation, multiple cytospin preparations may be made from the transport media, ideally at a concentration of 250 to 300 cells per microliter (cells/µL), allowing the cells to spread out and have adequate space separating the cells (Figure 29-5).12 Cells from transport media may also be placed in cell blocks. Multiple techniques are available for formation of cell blocks, and kits are available for the process.6,13 The main limitation of cell blocks is the use of formalin, which necessitates the use of antigen retrieval.6,14 Cytospin preparations from effusions and urine may also be prepared in a similar manner to that for cytospins from the transport media. One of the main advantages of submitting transport media or an effusion for ICC is the ability to make multiple slides from one sample, allowing for a panel of antibodies to be used rather than just one antibody. Using a combination of possible negative and positive antibodies will result in higher diagnostic yield than the use of one antibody.6,15 Each of these methods of sample preparation has advantages and disadvantages (Table 29-1). When submitting a sample, the sample should be sent overnight, and if transport media are being used, the sample should be sent on ice.