Chapter 19
The Pancreas
Cytologic evaluation of the pancreas is becoming increasingly common as a tool to help clinicians distinguish between pancreatic disorders. This increase in pancreatic aspiration for cytologic evaluation may be related to an increase in clinician comfort with pancreatic manipulation and the ongoing utility of pancreatic aspiration with minimal complications in human medicine. Previous perceptions that pancreatic manipulation may result in secondary pancreatitis have largely been unsubstantiated. A recent study showed that fine-needle aspiration (FNA) of normal canine pancreas does not result in increased serum concentrations of trypsin-like immunoreactivity (TLI) or canine-specific pancreatic lipase (cPL).1 However inter-operative biopsy was associated with increased serum TLI and sporadic, mild, peracute necrosis, inflammation, hemorrhage, and fibrin deposition.1
Currently, no single test conclusively differentiates inflammatory, cystic, neoplastic, and infectious diseases involving the pancreas. Patients with pancreatic disorders, with the exception of pancreatic insufficiency, often have similar histories and clinical signs. Clinicopathologic testing can frequently identify the presence of pancreatic disease in the dog.2,3 However, in the cat, serum chemistry tests are often less useful.4–8 In both dogs and cats, abdominal ultrasonography is a useful diagnostic tool to visualize and assess an abnormal pancreas, guide aspirates and biopsies, and monitor response to treatment.6, 9–11 As with biochemical tests, however, the use of abdominal ultrasonography to definitively differentiate between pancreatic diseases has variable sensitivity and specificity, as ultrasonographic appearance in various pancreatic disorders overlap.6, 9–11 Once visualized, ultrasound-guided FNA of the pancreas is a safe and effective adjunct to imaging in the diagnosis of pancreatic disorders. The pancreas exfoliates well, and cytologic examination of the pancreas in small animals has proved useful in the diagnosis of both neoplastic and nonneoplastic lesions, including abscesses, cysts, and pancreatitis.
Normal Pancreas Structure
Anatomy and Histology
The pancreas consists of a right (duodenal) limb and a left (transverse or splenic) limb joined at the head. The number and position of the pancreatic duct(s) opening into the duodenum and the location of the duct(s) to the common bile duct varies among species and among individuals in each species.12 The pancreas consists of endocrine and exocrine components. Numerous tubuloacinar secretory units form the exocrine component of the organ (Figure 19-1). These secretory units drain into long, narrow intercalated ducts lined by elongated, cuboidal cells. Intercalated ducts communicate directly with interlobular ducts.13 Functionally, the tubuloacinar secretory units (exocrine pancreas) secrete digestive enzymes in an inactive proenzyme form. Pancreatic enzymes are activated by trypsin secreted by the duodenum.
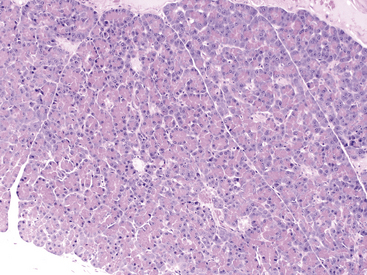
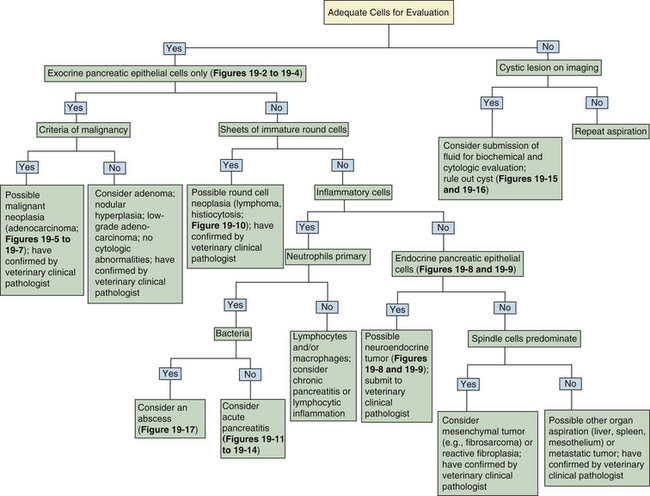
Figure 19-1 Histologic section of normal canine pancreas.
Normal pancreas consists of numerous lobules separated by septa of connective tissue. Lobules are primarily composed of tubuloacinar secretory units which form the exocrine component of the organ. Acinar cells have small, dark, uniform nuclei with abundant bright-pink cytoplasm. Islets and scattered intercalated ducts are also present that drain the epithelial cells. (Hematoxylin and Eosin stain. Original magnification 100×.)
The endocrine islets of Langerhans are clusters of epithelial cells scattered among the secretory units (Figure 19-2). Normal pancreatic islets contain four cell types, each secreting different pancreatic polypeptides: (1) α-cells secrete glucagon; (2) β-cells secrete insulin, (3) D-cells secrete somatostatin, and (4) F-cells secrete pancreatic polypeptide. β-cells are the most numerous islet cells (constituting 60% to 70% of the islet cells), and they are generally concentrated in the central part of the islet. α-cells constitute about 20% of the islet cells and are generally located peripherally.12
Figure 19-2 Histologic section of normal canine pancreas.
A pancreatic lobule composed of exocrine tubuloacinar secretory units with a single duct and a single aggregate of pale pancreatic endocrine cells (islet of Langerhans cells) near the center of the section. Acinar cells have a distinct polarity with centrally located eosinophilia (zymogen granules) and basilar basophilia (nucleus and cytoplasm). Scattered small vessels and small intercalated ducts are also present. (Hematoxylin and Eosin stain. Original magnification 100×.)
Sampling Technique
Methods
In veterinary medicine, percutaneous, ultrasound-guided FNA of the pancreas is the most common method of tissue sampling, although intraoperative sampling may also be performed.14 FNA of the pancreas permits extensive sampling and, in humans, is associated with a low risk of morbidity and mortality. It is especially useful in discriminating between pancreatic neoplasia and inflammation. In human medicine, FNA is the diagnostic method of choice for patients with a pancreatic mass. The most common methods of sample procurement are computed tomography (CT)–guided or endoscopic ultrasonography–guided aspiration. The vast majority of human pancreatic masses are neoplastic; as such, FNA is used to establish a rapid tissue diagnosis before chemotherapy, surgery, or both. In dogs, dynamic CT has recently been used to assess normal and neoplastic lesions of the pancreas.15
Methods to obtain a FNA of the pancreas are outlined in Box 19-1. The described methods for sample procurement are closely based on methods originally published by Bjorneby and Kari.14 In brief, the pancreas and surrounding abdominal structures should be thoroughly evaluated with ultrasonography to visualize the area to be aspirated. If a mass is present, multiple areas within the mass and surrounding tissue should be aspirated. In dogs, inflammation (purulent or lymphocytic) has been shown to occur in discrete areas throughout the pancreas (right and left limb).14 Therefore, no site is considered preferential to sample the pancreas (and confirm pancreatitis) in the absence of a visible lesion. The following steps are then performed:
• Label clean, glass slides, preferably with frosted edges, with patient identification and site of aspiration.
• Draw 1 milliliter (mL) of air into a 3-mL syringe. Attach a 1½- to 3-inch 22-gauge needle to the syringe. This needle-and-syringe combination may permit more accurate needle placement and angle control.16
• Using an ultrasound biopsy guide or freehand technique, place the needle in the desired region for aspiration, and move the needle back and forth within the pancreas.
• Be sure to identify the location of the needle with ultrasonography at all times, and maintain the needle in the same tract. For sample procurement, no additional negative pressure is required. Do not attempt to redirect the needle because the tip of the needle may lacerate the tissue and cause excessive hemorrhage and leakage of pancreatic enzymes.16
• To minimize cell disruption, sample expulsion and smear preparation should be as gentle as possible.
• To ensure full evaluation of the cells present, expel the sample onto the middle of the slide where cells are most readily stained and visualized.
• Sample three to four different sites within the lesion, if possible.
• To ensure the best-quality sample (and, thus, enhance the likelihood of a cytologic diagnosis), make multiple smears using a variety of smear techniques that result in both thin and thick preparations.
• Slide preparation techniques include the squash smear (slide-over-slide) or blood smear technique. The smears should be air-dried and submitted to a veterinary clinical pathologist.16
Troubleshooting
In general, pancreatic tissue exfoliates well for FNA. In case of any concerns with regard to sample quality, a cytopathologist should be consulted about sample attainment and preparation. Ruptured cells may be the result of negative pressure in the syringe while aspirating or too much pressure on slides while making preparations.16 Rapid drying of slides reduces refractile artifact on the slides. Hemodilution is common and generally will not confound diagnosis. However, if hemodilution is obscuring the diagnosis (especially distinguishing between blood contamination and inflammation), the number of times the needle is moved within the pancreas should be decreased. However, the tradeoff may be poor cytologic yield, which may occur if the needle biopsy technique is not aggressive enough. If clots tend to form, the needle and syringe should be flushed with an anticoagulant (i.e., ethylenediaminetetraacetic acid [EDTA]) prior to organ aspiration. Nondiagnostic samples because of poor cellularity may occur when lesions are fibrous or if the lesion was missed during aspiration. Nondiagnostic samples should be interpreted in light of imaging findings. Reaspiration may be attempted if the pancreas appears active and enlarged. However, if fibrosis is likely, intraoperative biopsy will likely be superior for obtaining a diagnosis. Cytologic findings should always be interpreted in light of imaging, physical examination, and biochemical findings. For example, if poorly cellular, proteinaceous fluid is obtained and the lesion on imaging is compatible with a cyst, then further diagnostics may not be warranted (Figure 19-3). However, if poorly cellular, proteinaceous fluid is obtained and the lesion is primarily solid or infiltrative with cystic or necrotic areas, reaspiration may be indicated because the primary lesion may not be represented.
Complications
Significant adverse effects secondary to percutaneous FNA of the pancreas in dogs or cats have not been reported. Rarely, FNA of the pancreas, in human beings, has been reported to cause complications, such as needle tract seeding of tumors, fistula formation, and ascites.14 In one human study, complications arising from FNA of both solid and cystic lesions of the pancreas were noted in only 4 of 248 (1.2%) patients. These complications, which included acute pancreatitis and aspiration pneumonia, were noted only after aspiration of cystic lesions.17 Recommendations from this study included avoiding needle passage through the main pancreatic duct or branch ducts dilated proximal to an obstruction. In addition, aspiration was terminated if blood became visible in the syringe or if there was obvious hemorrhage within the target lesion.17
Cytologic Evaluation
Normal
A decision tree to help guide initial cytologic evaluation of the pancreas is depicted in Figure 19-3. Exocrine epithelial cells are the most common cell type found on cytologic specimens from the pancreas. The background of the slide may contain blood from iatrogenic contamination, or it may be light pink, indicating a small amount of protein. Normal exocrine epithelial cells are found in small clusters to large sheets that may form tubular and acinar structures (Figure 19-4). On low magnification, the cytoplasm appears grainy with a pink hue because of the presence of small, pink zymogen granules. Unlike intestinal epithelial cells, cell-to-cell junctions are not prominent, giving cells a more indistinct, fluffy appearance (Figure 19-5). The cells are polyhedral, with abundant cytoplasm and a low nuclear-to-cytoplasmic ratio (Figure 19-4 Figure 19-5, and Figure 19-6). Nuclei are basilar in location, uniform, and round to oval. Chromatin is stippled to reticulate and a single, small, occasionally prominent nucleolus may be noted (Figure 19-5 and Figure 19-6). On high magnification, abundant pink, cytoplasmic granules, most consistent with membrane-bound zymogen granules, are noted. In preparations with abundant cell rupture, these granules may fill the background of the slide, giving a mottled blue-and-pink appearance (Figure 19-6). In a FNA of normal pancreas, no other cell populations will be present in high numbers. Occasionally, hematopoietic precursors, indicative of extramedullary hematopoiesis, small ductal cells, or uniform pancreatic endocrine cells, will be seen. The number of leukocytes present in the aspirate should be interpreted in light of peripheral blood cell counts to avoid interpreting peripheral neutrophilia or lymphocytosis as pancreatic inflammation.
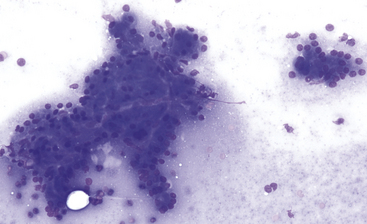
Figure 19-4 Fine-needle aspirate of normal canine pancreas.
Exocrine epithelial cells predominate and often exfoliate in small sheets and large clusters with acinar and tubular formations. Cells are polyhedral, cytoplasm is abundant, nucleus-to-cytoplasm ratios are low, and nuclei are uniform. (Wright-Giemsa stain. Original magnification 200×.)
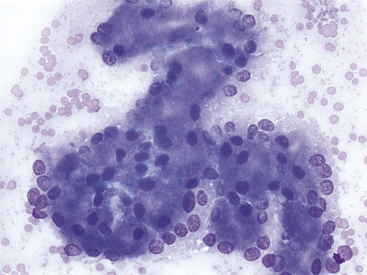
Figure 19-5 Fine-needle aspirate of normal feline pancreas.
The nuclei of exocrine epithelial cells often show a basilar distribution. Clear cell-to-cell junctions are not apparent. Nuclei are round to oval, chromatin is stippled, and single, small, uniform nucleoli can be present. (Wright-Giemsa stain. Original magnification 500×.)
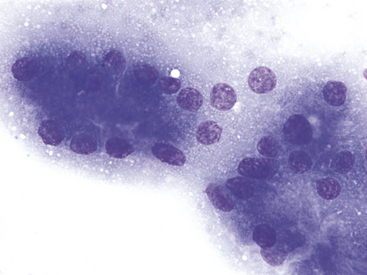
Figure 19-6 Fine-needle aspirate of normal canine pancreas.
Exocrine epithelial cells are characterized by apical, abundant, small, pink cytoplasmic granules, most consistent with membrane-bound zymogen granules. These granules give densely packed pancreatic exocrine epithelial cells a pink hue at lower magnification. (Wright-Giemsa stain. Original magnification 1000×.)
Pancreatic Lesions
A summary of the World Health Organization (WHO) scheme for histologic classification of pancreatic lesions of domestic animals is summarized in Box 19-2.18,19
Neoplasia
Adenoma
Benign exocrine epithelial tumors (i.e., exocrine adenomas, ductal [tubular] adenomas, or acinar adenomas) are rare in small animals and far less common than their malignant counterparts. They are generally small, solitary lesions found incidentally on imaging or necropsy examination. Histologically, they are partially or totally encapsulated, distinguishing them from the more common lesion of nodular hyperplasia.12,18,20 Cytologically, adenomas cannot be distinguished from normal or hyperplastic pancreatic tissue (Figure 19-3). If abundant, uniform, pancreatic exocrine epithelial cells are noted along with imaging findings suggestive of a solitary, solid lesion, differential diagnoses should include an adenoma, well-differentiated carcinoma, or a hyperplastic nodule (Figure 19-3).
Adenocarcinoma
Malignant tumors of the exocrine pancreas (i.e., adenocarcinomas, ductal [tubular] adenocarcinomas, or exocrine carcinomas) are rare in dogs and cats, with incidences of 17.8 in 100,000 patient years at risk in dogs and 12.6 in 100,000 patient years at risk in cats.20,21 This is in contrast to human medicine, in which pancreatic malignant tumors are the fifth leading cause of cancer-related deaths in the United States, with ductal adenocarcinomas accounting for more than 90% of these malignancies.21 The histiogenesis of adenocarcinomas in small animals remains uncertain. One author suggests that a ductular origin is likely based on tubular architecture, but ultrastructural analysis indicates that acinar cells may be the originator cell type, whereas other authors suggest that they may arise from either ductular or acinar epithelium, and often have features of both.12,20
In dogs, an increased incidence of pancreatic adenocarcinoma is seen with aging, and Airedale Terriers, Boxers, Labrador Retrievers, and Cocker Spaniels may be at increased risk.21 In one study of 13 dogs and cats, the average age at diagnosis was 9 and 10 years of age for dogs and cats, respectively.16 In another study, 85% of the dogs and cats with pancreatic adenocarcinoma had distant metastases at the time of diagnosis, and 88% of the patients had metastatic disease at the time of necropsy.16 Common metastatic sites include abdominal or thoracic lymph nodes, mesentery, adjacent gastrointestinal (GI) organs (including liver, duodenum, and jejunum), lungs, and less frequently, spleen, kidney, and diaphragm.16 Local, destructive infiltration may destroy the common bile duct.
Clinical signs at the time of presentation are nonspecific, but weight loss, vomiting, abdominal pain, and anorexia are common. Jaundice and cholestasis may result from obstruction of the bile duct by tumor, secondary liver disease, or both. Clinicopathologic tests may show increases in pancreatic enzyme activity, but evidence of extrahepatic biliary obstruction, including elevations in alkaline phosphatase (ALP) and alanine aminotransferase (ALT) activities, is more frequently seen. A peripheral neutrophilia is often noted.16 Described paraneoplastic syndromes include alopecia, exocrine pancreatic insufficiency, and cutaneous and visceral necrotizing panniculitis and steatitis.22–24
In dogs, pancreatic tumors frequently produce a mass, often in the midportion of the pancreas. In cats, tumors may be more diffuse and resemble nodular hyperplasia or chronic pancreatitis. Leakage of proteolytic enzymes from adenocarcinomas may be corrosive and result in cystic change in the primary tumor and necrotizing steatitis in the omental and peritoneal fat.20 Histopathologically, pancreatic adenocarcinomas show a tremendous range of differentiation. Some are well-differentiated tubular adenocarcinomas that form acinar structures, whereas others may form more solid sheets of poorly differentiated cells that no longer resemble pancreatic acini (Figure 19-7). They may be associated with a dense supporting stroma with a resultant scirrhous reaction. Focal hemorrhage and necrosis may occur along with focal accumulations of inflammatory cells, including T-lymphocytes.20
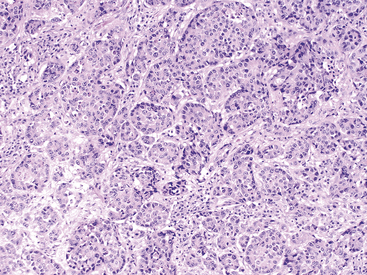
Figure 19-7 Histopathologic section of pancreatic carcinoma in a dog.
The section is hypercellular and consists of variably disorganized acinar epithelial cells found in islands and primitive tubules. Neoplastic islands are separated by desmoplastic stroma (mesenchyme). A general loss of uniform acinar architecture occurs. Anisocytosis and anisokaryosis are noted along with increased nuclear to cytoplasmic ratio and large, pale nucleoli. (Hematoxylin and Eosin stain. Original magnification 100×.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree