Chapter 4
Round Cells
• Cell shape: As was noted above, these cells are typically round to oval in shape; however, an amoeboid or irregular shape may be seen particularly with cells of histiocytic lineage.
• Nuclear shape: Cell nuclei of discrete round cell neoplasms are generally round to slightly oval; however, irregularly shaped nuclei are often present within the histiocytic group of discrete round cell neoplasms.
• Cytoplasmic characteristics: The cytoplasmic features of the various discrete round cell neoplasms are among the most important morphologic features used to differentiate one from the other. The presence or absence of cytoplasmic vacuoles, the presence or absence of cytoplasmic granules, and the color and distribution of cytoplasmic granules prove extremely helpful in making a definitive diagnosis.
• Tissue distribution: Discrete round cell neoplasms can be found essentially in any anatomic location, including both cutaneous and visceral tissue distributions. Some have relatively consistent locations of origin, which will be discussed later with each neoplasm type, and other chapters will highlight various visceral locations for discrete round cell neoplasms.
• Biologic behavior: Discrete round cell neoplasms have dramatically variable biologic behaviors. In many instances, although cytomorphologic atypia may be seen, these morphologic features often do not prove helpful in predicting potential biologic behavior.
The list of processes included in the discrete round cell neoplasm varies in the literature because of either including or excluding melanocytic neoplasms from the group. Since many of the cutaneous melanocytic neoplasms have a prominent round cytologic presentation, these are often included. A brief comment on the clinical and cytologic presentation of melanoma is included in this chapter. The entire group of discrete round cell neoplasms is as follows:1–6
Transmissible Venereal Tumors
Presentation and Biologic Behavior
Transmissible venereal tumors (TVT) were the first described transplantable neoplastic process. They are more commonly seen in more tropic urban areas, where large numbers of free-roaming dogs are present. Young sexually active dogs are most commonly affected because of direct contact and transplantation of neoplastic cells; external genitalia are most commonly affected. The typical social behavior of dogs licking and sniffing results in transplantation in other locations, including the oral and nasal cavities. The TVT is considered mostly to be a benign neoplastic process, which, with the aid of a competent immune system, will often spontaneously regress; however, metastatic disease, particularly to the intraocular space, may rarely occur.7,8
Cytologic Presentation
Aspirates and touch preparations of a TVT generally yield a large number of neoplastic cells. The typical TVT cell is the epitome of the discrete round cell neoplasm group. The distinctive discrete round cells have eccentric round nuclei with uniform granular chromatin patterns and, often, a single round prominent nucleolus. Mitotic figures are common and abnormal mitotic figures may be present, but these abnormal figures are not useful in predicting biologic behavior. Neoplastic cells range from 12 to 24 micrometers (µm) in diameter, and they have moderate amounts of granular and moderately blue-staining cytoplasm. Distinguishing cytoplasmic granules are not present; however, low-to-moderate numbers of TVT cells have distinguishing, clear, distinct, punched-out cytoplasmic vacuoles. These vacuoles are commonly similar in size and arranged in a linear array along the inner surface of the cell membrane (Figure 4-1).5,9
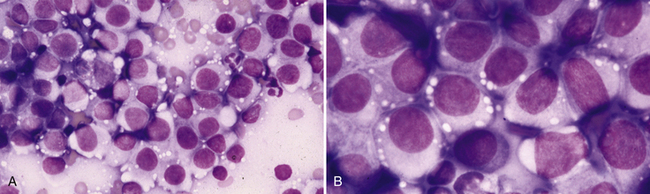
Figure 4-1 Transmissible venereal tumor. Aspirates of a transmissible venereal tumor on the prepuce of a dog.
A, Highly cellular specimen containing a monotonous population of discrete round mononuclear cells mixed with small amounts of peripheral blood. (Wright stain.) B, Higher magnification of a similar field of view demonstrating the discrete round nature of the cells, the eccentric round to slightly oval nuclei, the uniform chromatin patterns and the moderate amounts of pale blue cytoplasm with multiple, discrete, clear vacuoles at the periphery of the cell. (Wright stain.)
In addition to the neoplastic cells, normal-appearing small lymphocytes, few well-differentiated plasma cells, and rarely seen histiocytes or macrophages are commonly present.5 These cells tend to increase in relative numbers during spontaneous regression of the neoplastic process secondary to a localized immune response.
Mast Cell Tumors
Presentation and Biologic Behavior
Mast cell tumors are among the most common cutaneous neoplasms of dogs and cats; however, they may occur at any anatomic location both as primary and secondary neoplastic disease.8 They are most commonly seen in middle-aged dogs and cats, but an animal of any age is susceptible. In the dog, mast cell tumors are considered malignant, with the potential for widespread dissemination. A histologic grading scheme has been used to help predict biologic behavior; however, even well-differentiated grade I mast cell tumors have the potential for dissemination. Cytologic identification of metastatic or disseminated mast cell tumor, regardless of degree of differentiation, is of great value. MCT can also be found in the subcutaneous tissue and may be less aggressive than the cutaneous form. Histologic grading schemes for cutaneous mast cell tumors cannot be applied to assess the behavior of subcutaneous mast cell tumors.
The general features of the histologic grading system with canine cutaneous mast cell tumors are outlined next:5,7
Grade I: well-differentiated, generally well-defined, superficial, low mitotic index
Grade II: moderately differentiated, moderate to poorly circumscribed, mild to moderate infiltration into deeper dermal tissues, moderate mitotic index, potential slight cytomorphologic atypia
Grade III: potentially poorly differentiated, poorly circumscribed, deep infiltration into subcutis, potential high mitotic index, potential moderate cytomorphologic atypia
Feline mast cell tumor has multiple presentations. Isolated cutaneous mast cell tumor is generally considered a benign lesion in the cat, and complete excision typically proves curative; however, dissemination may even be seen in cases with suspected isolated cutaneous mast cell tumor.10–12 A guarded prognosis with short median survival times is reported in cats with multiple cutaneous mast cell tumors, recurring mast cell tumors and primary splenic mast cell neoplasia. Degree of differentiation is commonly considered a poor prognostic finding; however, the literature is contradictive with regard to the more poorly differentiated mast cell tumor in the cat. One study found no correlation between degree of cell differentiation and prognosis.1,13
Cytologic Presentation
The diagnosis of mast cell tumor in dogs and cats is relatively straightforward in most cases. Cytologic specimens are commonly very cellular and peripheral blood contamination is common. In addition to the distinctive discrete round cell characteristics, mast cell tumors have distinguishing fine-to-coarse purple granules when stained with a Romanowsky-type stain (Figure 4-2).1 These granules allow a definitive diagnosis in most cases; however, it is reported that one of the most commonly used stains in practice, Diff-Quik, often fails in staining these granules, making accurate identification of mast cell tumor from other discrete round cell tumors difficult or impossible. Anecdotal reports suggest that approximately 15% of canine mast cell tumors will not stain with Diff-Quik (Figure 4-3). In reality, if one examines the specimen very carefully, although staining may be significantly less with Diff-Quik, identifiable purple granules are able to be visualized. Additional anecdotal reports suggest that prolonged fixation of mast cell tumors in the first solution of the Diff-Quik stain improves staining of mast cell granules.
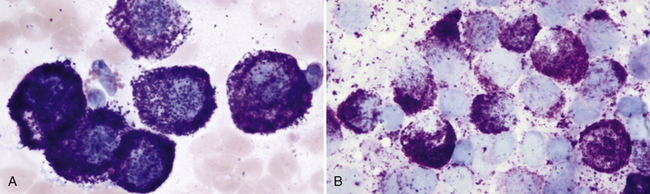
Figure 4-2 Mast cell tumor. Aspirates of two well-differentiated mast cell tumors on the skin of two different dogs.
A, Moderately cellular specimen with significant peripheral blood contamination, which is common with mast cell tumor aspirates. The neoplastic cells are uniform and well differentiated. Nuclei are almost obscured from view because of the many coarse purple granules. Note the one eosinophil in the center left of the field of view. (Wright stain.) B, Highly cellular preparation with densely packed sheets of mast cells, where neoplastic cell nuclei are stained very pale blue because of the heavy degree of granulation and lack of stain penetration to the nucleus. (Wright stain.)
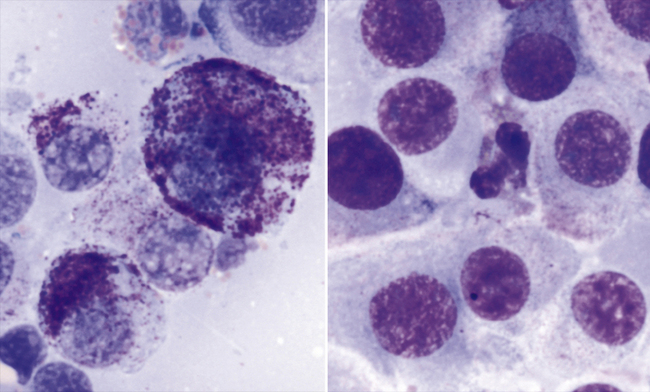
Figure 4-3 Aspirates of a cutaneous mast cell tumor on a dog.
The image on the left is from a Wright-stained preparation, and the image on the right is from a Diff-Quik–stained preparation from the same aspirate. On the basis of the Wright-stained preparation (left), the tumor is characterized as moderately well differentiated with neoplastic cells containing variable numbers of fine to coarse, purple to pink granules. On the Diff-Quik–stained preparation (right), only extremely few cytoplasmic granules are distinguishable. The eosinophil in the center of the field of view gives a possible clue to an underlying poorly differentiated mast cell tumor, or one of those mast cell tumors that do not stain well with Diff-Quik stain.
Although canine mast cell tumor grading is based on histologic evaluation, where comments on degree of tissue invasion becomes an important criterion, degree of differentiation of mast cell tumor cells is possible cytologically, and correlation to histologic grading is good. The less differentiated cells are less granulated and have greater variability of cell size, nuclear size, and nucleus-to-cytoplasm (N:C) ratios. Prognosis should be considered guarded for the less differentiated canine neoplasms; however, it should be noted that well-differentiated mast cell tumors may also disseminate. Identification of metastasis to regional lymph nodes or parenchymal organs proves most useful in characterizing the biologic behavior of mast cell tumors in dogs and cats (Figure 4-4). With feline mast cell tumors, the distribution of neoplasms (isolated, multiple or disseminated) and the degree of circumscription of the neoplasm are potentially more predictive of biologic behavior compared with degree of differentiation (Figure 4-5).
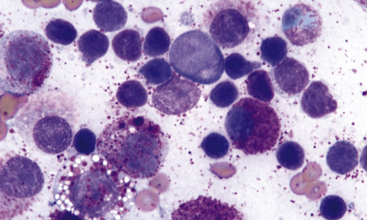
Figure 4-4 Aspirate of a popliteal lymph node from a cat with multiple cutaneous mast cell tumors.
Note the mixture of moderately well-differentiated mast cells mixed with many normal-appearing small lymphocytes, which represent a population of residual normal lymphoid elements in the lymph node. A single eosinophil is noted in the top right region of the field of view; eosinophils are not as commonly seen in feline mast cell tumor as in the canine counterpart. (Wright stain.)
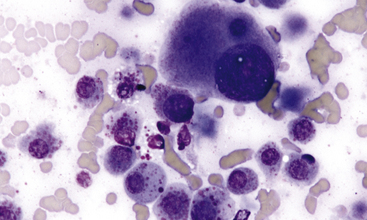
Figure 4-5 Aspirate of an isolated and well-defined cutaneous mast cell tumor in the skin of a cat.
Intermixed with moderate amounts of peripheral blood, many poorly granulated mast cells are present. Marked cytomorphologic atypia is seen; however, on complete surgical excision, this animal was disease free with no recurrence of neoplastic disease. (Wright stain.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







