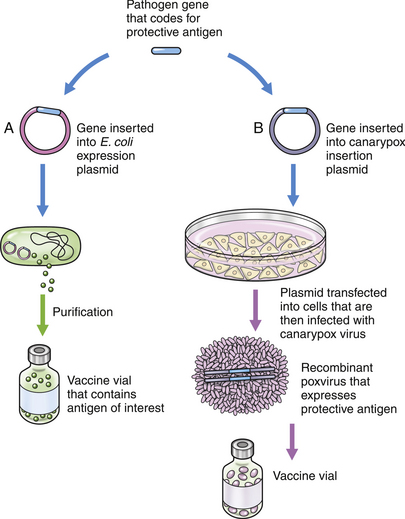
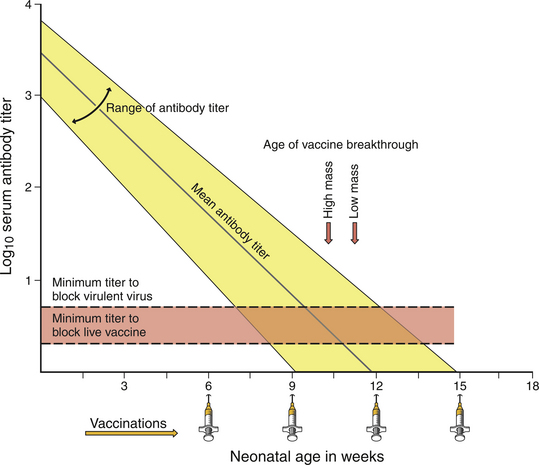
Chapter 12 • Active immunization can partially or completely protect dogs and cats from severe consequences of infection with a variety of different pathogens, and in some cases it reduces shedding of these pathogens. • Vaccines contain attenuated live microorganisms, inactivated microorganisms, or portions of these organisms. They also contain preservatives and adjuvants. • Failure of immunization can occur with improper storage or administration of vaccines, a large challenge dose, host factors such as concurrent infections or disease, and interference by maternal antibody. • Other adverse effects of vaccine administration are uncommon to rare but include hypersensitivity reactions, disease induced by live attenuated vaccine organisms, and injection-site sarcomas in cats. • The decision to administer a vaccine should be based on discussion of risks and benefits between the veterinarian and pet owner. This should be documented in the medical record. • Guidelines for vaccine selection and administration have been published by a number of veterinary bodies, such as the AAFP, AAHA, AVMA, and WSAVA; suggestions can also be found in Appendix I. Immunization refers to artificial induction of immunity or protection from infectious disease and may be active or passive. Active immunization involves administration of vaccines that stimulate cell-mediated or humoral immunity, or both, to a specific pathogen. Passive immunization refers to the administration of antibodies in order to provide temporary protection from disease and can occur through acquisition of maternally derived antibody (MDA) transplacentally, in colostrum, or milk; or treatment with preparations that contain specific or nonspecific immunoglobulins (see Immunomodulators, Chapter 7, and post-exposure prophylaxis for rabies, Chapter 13). Readers are referred to advanced immunology texts for detailed descriptions of the physiology of active and passive immunity.1 The goal of immunization is to generate a protective immune response of prolonged duration against a specific infectious disease, with minimal adverse effects. Because of the potential for adverse effects, vaccination should be performed only if there is a risk for significant morbidity or mortality from an infectious disease. Since the 1950s, a huge number of vaccines for dogs and cats have been developed and marketed worldwide, and more are in development. Nevertheless, it is estimated that even in developed countries such as the United States, only 30% to 50% of dogs are properly immunized, and possibly an even smaller proportion of cats.2,3 Appropriate vaccination of a larger proportion of the pet population may assist in reduction of the prevalence of infectious diseases through the induction of herd immunity. A vaccine is a suspension of attenuated live or inactivated microorganisms, or parts thereof, that is administered to induce immunity. In addition to protective antigens, vaccines may contain preservatives and stabilizers as well as specific antibiotics to preserve the antigen and inhibit bacterial and fungal growth within the vaccine. Some vaccines also contain an adjuvant to enhance the immune response to the antigen. Although the mechanisms are not completely clear, adjuvants can delay the release of antigen from the site of injection and induce the secretion of chemokines by leukocytes.4 The most widely used adjuvants are particulate adjuvants, such as those that contain aluminum salt precipitates such as aluminum hydroxide.5 Other particulate adjuvants include immunostimulators such as saponin, which is present in a canine Leishmania vaccine. Attenuated live vaccines (or modified live vaccines) contain microorganisms that are artificially manipulated so as to negate or greatly reduce their virulence, or are field strains of low virulence. Repeated passage through cell culture is the most common means of attenuation. Because they replicate in the host, organisms in attenuated live vaccines usually stimulate an immune response that most closely mimics the protection that results from natural infection. Vaccination with attenuated live canine parvovirus (CPV) and canine distemper virus (CDV) vaccines in the absence of MDA can result in protective immune responses within 3 days of a single injection, which may be followed by immunity that lasts many years, if not for life.6–8 Partial immunity after vaccination with attenuated live CDV and feline panleukopenia virus (FPV) vaccines can occur within hours.3,9,10 In addition, vaccine organisms that are shed can serve to immunize other animals in a population. However, the potential for reversion to virulence or vaccine-induced disease exists. Vaccine-induced disease is most likely to occur in highly immunosuppressed animals. Attenuated live vaccines also have the potential to cause some immunosuppression in their own right,11,12 or they may shift the balance from Th1 to Th2 immune responses.13 Rarely, this can lead to clinical disease. For example, an outbreak of salmonellosis was reported in cats after use of a high-titered attenuated live FPV vaccine.14 Very rarely, contamination of attenuated live vaccines has occurred with other pathogenic microorganisms present within cell cultures used to propagate the vaccine. Generally speaking, inactivated vaccines are less effective than attenuated vaccines, because replication in the host does not occur. They produce weaker immune responses of shorter duration, and more frequent booster immunizations may be required. Two initial doses of vaccine 3 to 4 weeks apart are essential to produce an effective immune response, and if more than 6 weeks elapses between these doses, it has been recommended that the series should be repeated.15 Beyond the initial vaccination series, it is not clear whether lapsed annual boosters require the series to be restarted. This is not considered necessary for human immunization16 but has been suggested for dogs when more than 2 or 3 years elapses between boosters.15 Inactivated vaccines usually contain adjuvant as well as a large infectious dose to improve immunogenicity. They are safer than live attenuated vaccines for use during pregnancy and in very young or debilitated animals. Although bacterins have traditionally been associated with a greater likelihood of allergic reactions than live attenuated vaccines, newer inactivated vaccines are safer and have reaction rates that more closely approach those of live attenuated vaccines. The maximum duration of immunity that is induced by commercially available bacterins for dogs and cats remains largely unknown, partly because challenge studies that evaluate long-term duration of immunity are prohibitively expensive. However, some inactivated viral vaccines have been shown to have durations of immunity in excess of 7 years in cats.17 Caution is required when extrapolation is made from the duration of immunity for one product to that for a similar product from a different manufacturer, because it may not be equivalent. Although bacterins usually do not protect all animals from infection, they may prevent clinical illness. In some cases, natural infection of vaccinated animals serves to further boost the immune response, and this can influence duration of immunity in the field. The advantages and disadvantages of attenuated live and inactivated vaccines are shown in Table 12-1. TABLE 12-1 Advantages and Disadvantages of Attenuated Live and Inactivated Vaccines 1. Recombinant subunit vaccines. These are produced by cloning one or more genes for a protective antigen into an expression vector, such as in Escherichia coli. The protein expressed by the bacteria is then purified and used in the vaccine (Figure 12-1, A). An example of a recombinant subunit vaccine is the Lyme recombinant OspA vaccine. FIGURE 12-1 Examples of recombinant DNA vaccines. A, Recombinant subunit vaccine. The gene of interest is inserted into an expression vector such as a plasmid taken up by Escherichia coli, which subsequently produces large amounts of an immunogenic protein. This is purified and used in the vaccine. B, Vectored vaccine. The gene or genes of interest are inserted into a canarypox or vaccinia vector, which is then inoculated into an animal. Replication of the vector within the host is followed by expression of the immunogenic protein. 2. Deletion mutant vaccines. These are produced by deleting virulence genes from a pathogen while protective antigens are left in place. There are currently no such vaccines for dogs and cats. 3. Vectored vaccines. These are produced by inserting genes for one or more protective antigens into the genome of a virus. The virus replicates in the host and expresses the antigens but is nonpathogenic (see Figure 12-1, B). Currently available vectored vaccines for dogs and cats use canarypox virus as a vector. 4. DNA vaccines. These consist of naked DNA that encodes the antigens required for protective immunity. The DNA is injected directly to the animal using an inoculation system. The DNA is then taken up by host cells and translated into antigen. Both humoral and cell-mediated immune responses are produced. DNA vaccines are not currently available commercially for use in dogs and cats. Vaccines should be stored and administered according to label recommendations. Inactivation of vaccines can occur if they are inadvertently frozen or heated to excessive temperatures, exposed to excessive amounts of light, or used beyond their expiration date. Hands should be washed before preparation and administration of the vaccine. Lyophilized products should be reconstituted with the proper diluent, and different vaccines should not be mixed in the same syringe or vial. Reconstituted products should be used immediately. It has been recommended that attenuated live vaccines be discarded if more than 1 hour has lapsed since reconstitution,15 although no published reports exist of the viability of vaccine organisms over time after reconstitution or of the ability of stored, reconstituted vaccine to elicit an immune response. Vaccines should only be used in the animal species for which they are labeled, or serious adverse effects or failure of immunization can occur. If vaccines for multiple different pathogens are to be administered simultaneously, they should be injected at distant sites or, if possible, a combination vaccine should be used. Simultaneous vaccination for more than one pathogen does not appear to interfere with immune responses to each component of the vaccine,18–20 and vaccine manufacturers must demonstrate that the protection that occurs for a specific pathogen after vaccination with a combination product equals the protection that occurs when a vaccine for only that pathogen is given. In contrast, successive parenteral administration of different attenuated live vaccines at 3 to 14 day intervals has the potential to interfere with immune responses. An interval of 4 weeks is preferred for human patients.16,21 Inactivated vaccines do not produce interference in this way.16 If possible, administration of vaccines to animals that are under anesthesia should be avoided because adverse reactions may be difficult or impossible to recognize in this situation. It is not necessary to re-administer an intranasal vaccine if the animal coughs or sneezes after administration. The site and route of administration, product, serial number, expiry date, and individual who administered the vaccine should be recorded for each vaccine administered.2 Vaccine vials often possess adhesive labels that can be easily removed and applied to a paper medical record. The immune response is divided into innate and adaptive immune responses. The innate immune response is nonspecific and acts as an immediate line of defense against an infection. Components of the innate immune response consist of natural killer cells, which recognize host cells that are infected by viruses; complement, which is activated by bacterial cell wall components; and phagocytes, such as macrophages and dendritic cells. The adaptive immune response develops over several days and involves presentation of antigen by dendritic cells in association with the major histocompatibility complex and stimulation of B and T cell responses, together with the formation of memory B cells. The nature of the innate response influences the subsequent adaptive response. Cells of the innate immune system possess pattern recognition receptors that can recognize patterns that are characteristic for various pathogens (pathogen-associated molecular patterns, or PAMPs), including Toll-like receptors and NOD-like receptors. PAMPs are under investigation for use as adjuvants in human and animal vaccines in order to create improved T cell immune responses.4,22 The ability of a vaccine to induce an immune response depends not only on the target pathogen, vaccine composition, and route of administration, but also on host factors such as age, nutrition, pregnancy status, stress, concurrent infections, and immune status, including the presence or absence of passively acquired antibody (Box 12-1). Some of these factors may also influence vaccine safety. Some animals, particularly dogs of the Rottweiler breed, may have an impaired ability to respond to vaccination. These dogs have been termed nonresponders.2,23 This situation is probably rare if efficacious vaccines are used and booster vaccines are administered. Young dogs, less than 1 year of age, have a significantly reduced response to vaccination with rabies virus vaccines when compared with adult dogs.24 Small-breed dogs have a greater serologic response to rabies vaccines than large-breed dogs.25 Administration of vaccines to febrile animals or animals with moderate to severe illness should be avoided if possible until recovery has occurred, because the immune response to the vaccine may be suboptimal. In young animals, MDA can neutralize vaccine antigens and interfere with effective immunization. This is one of the most common reasons for vaccine failure in dogs and cats. Any MDA titer against CPV has the potential to interfere with immunization. The amount of MDA in a puppy or kitten at any one point in time cannot be predicted because it varies depending on the titer of the dam and the amount of colostrum ingested after birth. As a result, a series of vaccinations are administered in order to increase the chance that successful immunization will occur soon after the decline of MDA titers to sufficiently low concentrations (Figure 12-2). Nevertheless, a window always exists when MDA concentrations are high enough to interfere with immunization, but not sufficient to prevent natural infection. This window is known as the window of susceptibility or the window of vulnerability. The use of recombinant vectored vaccines can overcome the interference by MDA, although the extent to which this applies in animals that have passive immunity to the vector virus (i.e., immunity transferred from a dam that was immunized with a recombinant vector vaccine) requires clarification. Because replication of the vector is aborted, the immune response to the vector itself may be reduced. As a result, passive transfer of neutralizing antibody titers to the vector may not occur. Mucosal vaccines can also provide greater protection in the face of MDA; the mucosal immune system matures shortly after birth.26,27 FIGURE 12-2 Influence of maternal antibody (MDA) on immunization. Puppies and kittens acquire variable amounts of MDA transplacentally and through colostrum after birth. This binds to vaccine antigens and inhibits the immune response. A series of vaccines are administered to maximize the chance of inducing an immune response as MDA concentrations decline. The window of susceptibility is the period of time when MDA concentrations are high enough to interfere with immunization, but not sufficient to prevent natural infection. High antigen mass vaccines provide protection earlier than low mass vaccines. (From Greene CE, Schultz RD. Immunoprophylaxis. In: Greene CE, ed. Infectious Diseases of the Dog and Cat, 3 ed. St Louis, MO: Saunders; 2006.) For some vaccines, such as rabies, CDV, CPV, and FPV, the presence of circulating antibodies correlates with protection (Table 12-2). Thus, serologic assays have been used in dogs and cats to decide whether vaccination is necessary or likely to be effective. These serologic assays have also been used to clear pets for travel. TABLE 12-2 Antibody Titers That Correlate with Protection against Distemper, Parvovirus, and Rabies
Immunization
Introduction
Vaccine Composition and Types of Vaccines
Attenuated Live
Inactivated
Advantages
Rapid onset of immunity
Sustained immunity after single dose
May immunize others in populations
Improved breakthrough of maternal antibody interference
Safe, even in immunocompromised and pregnant animals
Do not interfere with development of immunity from other vaccines
Stable in storage
Disadvantages
Potential for reversion to virulence
Virulence in the immunocompromised
Contraindicated in pregnancy
May cause immune suppression
Can interfere with development of immunity if administered within days to 2 weeks of another vaccine
Less stable in storage
Potential for vaccine contamination
Slow onset of immunity
Multiple boosters required
Often highly adjuvanted, with greater potential for adverse effects
Reduced degree of protection compared with attenuated live vaccines
Poor breakthrough of maternal antibody interference
Vaccine Storage, Handling, and Administration
Components of the Immune Response
Determinants of Immunogenicity
Measurement of the Immune Response
Pathogen
Minimum Protective Titer
Methodology Used
Canine distemper virus
≥1:16 to 1:20
Serum neutralization (SN)
Canine parvovirus
≥1:80 to 1:100
Hemagglutination inhibition (HI)
Rabies
≥0.5 IU/mL
Fluorescent antibody virus neutralization (FAN) ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


