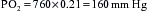CHAPTER 201 Hyperbaric Oxygen Therapy
Hyperbaric oxygen (HBO) is treatment with high-concentration oxygen achieved by having the patient breathe 100% oxygen inside a pressurized hyperbaric chamber. Oxygen is delivered to the tissues through respiration because absorption of oxygen through the skin is insufficient. HBO treatment is based on two physical factors related to the hyperbaric environment: mechanical effects of pressure and increased oxygenation of tissues. This chapter reviews the scientific and clinical literature regarding HBO therapy in laboratory animals and in humans and introduces the practitioner to the potential use of this treatment modality for the equine patient.
PHYSICAL PRINCIPLES OF HYPERBARIC THERAPY
Pressure
Pressure is measured as force per unit area. One atmosphere of pressure (ATA) is equal to 14.7 pounds per square inch (PSI), which results from the weight of the air on the surface of the earth at sea level. Weathermen usually refer to this pressure as barometric pressure, and quantify it in inches or millimeters of mercury or kilopascals (1 ATA = 29.9 inches Hg = 760 mm Hg = 10 kPa) (Table 201-1). The term atmosphere refers to atmospheres absolute. Absolute pressure equals the gauge pressure plus the ambient air pressure on the surface at sea level (1 ATA). For example, if one descends 33 feet in seawater, one is at an absolute pressure of 2 ATA. This is because 33 feet of water exerts a pressure of 14.7 PSI as read on the gauge. Absolute pressure at this depth equals gauge pressure plus atmospheric pressure (i.e., 1 ATA + 1 ATA = 2 ATA).