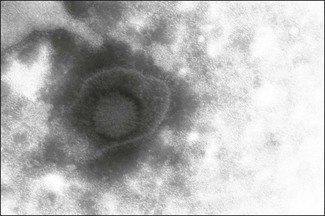
Chapter 49 The family Herpesviridae includes more than 100 viruses infecting fish, amphibians, reptiles, birds and mammals including man. Their ubiquitous occurrence, evolutionary diversity and involvement in a range of important medical and veterinary diseases make this group of viruses one of the most important. The name is derived from the Greek word ‘herpein’ meaning ‘creeping’ in reference to the recurrence of vesicles in people infected with herpes simplex virus. Herpesviruses are enveloped and range in size from 200–250 nm in diameter (Fig. 49.1). Virions contain linear, double-stranded DNA within an icosahedral capsid, approximately 125 nm in diameter. Between the envelope and the capsid there is a layer of amorphous material containing several proteins, termed the tegument. Herpesviruses enter cells by fusion with the plasma membrane. The capsid then moves to the nucleus where transcription, viral DNA replication and capsid assembly take place. The envelope is probably derived from the nuclear membrane of the host cell, although this is currently a matter of some debate. It is modified such that it contains several different viral-encoded glycoproteins. The enveloped virions accumulate in the endoplasmic reticulum prior to final processing of glycoproteins in the Golgi apparatus and release by exocytosis. Productive infection results in cell death. Intranuclear inclusions are characteristic of herpesvirus infection. Infected cells may fuse to form syncytia permitting virus to infect neighbouring cells without being exposed to antibody. Host protective antibody responses are usually directed against the envelope glycoproteins. Herpesvirus virions are fragile, sensitive to detergents and lipid solvents, and do not survive well in the environment. The family is divided into three subfamilies (Fig. 49.2) containing twelve genera; Alphaherpesvirinae, Betaherpesvirinae, Gammaherpesvirinae. A previously unassigned genus Ictalurivirus containing ictalurid herpesvirus 1, a virus of channel catfish, has now been assigned to a separate family Alloherpesviridae within the order Herpesvirales. The subfamily classification was originally based solely on biological properties but nucleotide sequence and phylogenetic analyses have resulted in some recent changes, most noticeably Marek’s disease virus which was originally classified as a gammaherpesvirus has now been placed in its own genus in the alphaherpesvirus subfamily. Alphaherpesviruses replicate and spread rapidly, destroying host cells and often establishing latent infections in sensory nerve ganglia. Betaherpesviruses replicate slowly, spread slowly and cause infected cells to become enlarged, hence the common name cytomegalovirus. They may become latent in cells of the monocyte series. Gammaherpesviruses are specific for T or B lymphocytes and can establish latent infections in these cells. However, infection of lymphocytes is frequently arrested with minimal expression of the viral genome. A number of gammaherpesviruses are associated with lymphoproliferative disorders such as human herpesvirus 4 (Epstein–Barr virus) which is associated with Burkitt’s lymphoma in children and human herpesvirus 8 which is associated with Kaposi’s sarcoma. A feature common to all herpesviruses is the ability to establish life-long latent infections which may be reactivated to cause further bouts of clinical disease (Table 49.1). Shedding of virus may be periodic, coinciding with recrudescence, or continuous. During latency the episomal viral genome becomes closed and circular with only a small subset of genes expressed. Reactivation of infection is associated with various stressors including transportation, overcrowding, intercurrent infection and adverse weather. In nature herpesviruses are usually restricted to a single host species. The viruses are highly adapted to their natural hosts as a result of co-evolution and infections are usually inapparent or trivial. In some circumstances, particularly in the very young, where there is immunosuppression or following infection of an alternative host, infections can be life-threatening. A number of herpesviruses, such as gallid herpesvirus 2 (Marek’s disease virus), have been implicated in neoplastic transformation. Table 49.1 Herpesviruses of veterinary significance • Ocular swabs and nasal or genital swabs rubbed vigorously against the mucosal surface and collected from several affected animals in the early acute phase of the disease are suitable for virus isolation (Nettleton et al. 1983). The virus is reasonably fragile and specimens should be submitted in viral transport medium at 4°C. Mucous membranes of the respiratory tract as well as lung and bronchial lymph node samples can be collected during post mortem examination of animals that have died due to the respiratory disease. Suitable foetal samples include liver, lung, spleen and kidney. A wide range of bovine cell lines are susceptible to infection including primary, secondary and continuous cell lines such as Madin-Darby bovine kidney cell line. The virus produces a rapid cytopathic effect (CPE) in bovine cell lines, usually within three days of inoculation, characterized by holes in the monolayer surrounded by grape-like clusters of rounded cells. Raw semen is toxic to cells and must be prediluted. • For a more rapid diagnosis it is possible to make smears from ocular, nasal or genital swabs or to cut frozen sections from the tissues of aborted foetuses in order to demonstrate the presence of viral antigen by immunofluorescence. Nasal samples should be obtained from a number of animals in the early stages of the respiratory disease in order to increase the chances of detection. Viral antigen can also be detected using ELISA (Edwards & Gitao 1987). These techniques are not as sensitive as virus isolation. • The presence of characteristic gross and microscopic lesions, particularly intranuclear inclusions, in aborted foetuses is highly suggestive and helpful in establishing a diagnosis of BoHV-1. • The polymerase chain reaction has been adapted for detection of BoHV-1 viral DNA in clinical samples (Moore et al. 2000) and is the method of choice for the detection of virus in semen (Smits et al. 2000). It is more sensitive and rapid than virus isolation and can also be used to detect latent infection in sensory ganglia. Assays based on detection of gE sequences can be used to discriminate between wild-type virus and gE-deleted vaccine strains (Fuchs et al. 1999). Real time PCR protocols have also been described (Kramps 2008). • The collection of paired sera and demonstration of a rising antibody titre by virus neutralization or ELISA (Kramps et al. 1993) is indicative of infection. Several ELISA kits are available commercially as well as gE-ELISAs that can be used in conjunction with marker vaccines to distinguish infected cattle from vaccinates (Wellenberg et al. 1998). The blocking ELISA format, particularly gB-specific ELISAs, is considered to be more sensitive than the indirect ELISA format (Perrin et al. 1993, Kramps et al. 2004). Serological testing to demonstrate a rising titre is less useful in abortion cases due to the delay between infection and time of abortion such that animals often have high titres at the time of abortion. The ELISA has been adapted for the testing of bulk milk samples for surveillance purposes (Nylin et al. 2000). Infected herds where less than 20% of the animals tested have antibodies to BoHV-1 will give a negative bulk milk test. Animals infected in early life when maternally derived antibodies are present may become latently infected but not develop an active antibody response (Lemaire et al. 2000). The failure to detect these seronegative latent carriers (SNLC) serologically, presents a difficulty in the implementation of control programmes. In endemically infected wildebeest populations alcelaphine herpesvirus 1 is transmitted both vertically and horizontally. Latent infection is thought to occur in lymphoid cells. In most cases infection is acquired by calves shortly after being born from their mothers or infected comrades via nasal secretions. Transplacental transmission occurs in some instances. A persistent viraemia occurs in young wildebeest for the first few months of life and it is at this time that large quantities of cell-free, infectious virus is shed in nasal and ocular secretions. Cattle in contact with wildebeest at this time may become infected. The situation in sheep is thought to be similar to that in wildebeest with the greatest risk of transmission to cattle associated with lambing time and contact with young lambs. However, a number of studies indicate that young sheep at six to nine months of age are the highest risk group for transmission (Li et al. 2004). Cattle and deer are considered to be ‘end-hosts’ as virus does not appear to be transmitted by infected animals. Both seroconversion and the presence of OHV-2 DNA have been reported in clinically normal cattle suggesting that cattle may become infected without developing clinical signs (Powers et al. 2005). The disease has been shown to also occur in pigs (Loken et al. 1998). The pathogenesis of MCF is not well understood. The presumed site of entry to the body is the upper respiratory tract. A cell-associated viraemia occurs but several studies have found little evidence of viral antigen in affected organs. For many years the cause of lesions had been ascribed to an autoimmune phenomenon characterized by a necrotizing process and destruction of normal host cells. However, recent evidence suggests that lesions are caused by the activities of virus-infected, dysregulated cytotoxic T cells (Russell et al. 2009). The incubation period is highly variable but typically lasts about three to four weeks. Four clinical forms have been described; peracute, ‘head-and-eye’, intestinal and mild. The ‘head-and-eye’ form is the most common. It is characterized by sudden onset, fever, ocular and nasal discharges, enlarged lymph nodes, conjunctivitis with corneal opacity and erosive mucosal lesions in the upper respiratory tract leading to profuse mucopurulent nasal discharge and encrustation of the muzzle. Some animals display central nervous signs including muscular tremors, incoordination and head pressing. Diarrhoea or dysentery may be a feature of the disease. The course of the disease is usually three to seven days, typically ending in death of the animal. However, chronic cases lasting several weeks or months as well as recovery have been described (O’Toole et al. 1997). In peracute cases, particularly in deer, there may be sudden death without premonitory signs. • Diagnosis is generally based on clinical signs and characteristic histopathological changes including disseminated fibrinoid vasculitis, widespread lymphoid infiltration and ulceration of surface epithelia. • In the clinically affected live animal detection of OHV-2 DNA by PCR is the test of choice and can be used with peripheral blood leukocytes, fresh tissues and paraffin-embedded tissues (Baxter et al. 1993, Muller-Doblies et al. 1998, Crawford et al. 1999). • Antibody to OHV-2 can be detected in 70 to 80% of clinically affected cattle by indirect immunofluoresence or the immunoperoxidase test using AlHV-1-infected cells. A competitive inhibition ELISA has been developed for the detection of serum antibodies to AlHV-1 and OHV-2 (Li et al. 1994, 2001). It is not as sensitive a test as PCR or histopathology for the diagnosis of MCF in clinically affected animals but is extremely useful in epidemiological studies. • Isolation of AlHV-1 in bovine thyroid cells is possible from the buffy coat and lymph nodes of animals with WA-MCF. Evidence of CPE may not be visible for up to 21 days postinoculation. Ovine herpesvirus-2 has not been isolated to date. Diagnosis is based on clinical signs and demonstration of virus in scrapings or vesicular fluid by direct electron microscopy or alternatively using virus isolation in primary bovine cell lines. The temperature of incubation for inoculated cell cultures should be reduced to 32°C. A PCR assay has been published that is suitable for the detection of BoHV-2 in skin lesions (d’Offay et al. 2003). Paired sera can be used to try to demonstrate a rise in antibody titre but a high titre of antibody is frequently already present by the time the first sample is obtained. Suitable serological tests include virus neutralization, complement fixation, agar gel immunodiffusion and indirect immunofluorescence.
Herpesviridae
Virus
Host
Significance of infection
Bovine herpesvirus 1
Cattle
Worldwide, important viral infection of cattle. Separate respiratory (infectious bovine rhinotracheitis) and genital (infectious pustular vulvovaginitis) forms of disease. Abortion occurs in pregnant cows following respiratory disease outbreaks
Bovine herpesvirus 2
Cattle
Infection in temperate regions associated with ulcerative mammillitis. Mild, generalized skin infection, pseudo-lumpy skin disease, described in tropical and subtropical regions
Bovine herpesvirus 4
Cattle
Frequently subclinical but has been associated with mastitis and endometritis
Bovine herpesvirus 5
Cattle
Associated with outbreaks of non-suppurative encephalitis in calves in USA, Argentina, Hungary and Australia
Alcelaphine herpesvirus 1
Wildebeest, cattle, deer
Subclinical infection in wildebeest. Associated with malignant catarrhal fever in cattle and deer. Distribution confined to Africa and zoological collections
Ovine herpesvirus 2
Sheep, goats, cattle, deer
Subclinical infection in sheep and goats. Associated with malignant catarrhal fever in cattle and deer. Worldwide distribution
Equine herpesvirus 1
Horses
Infection associated with abortion, respiratory disease, generalized neonatal infection and nervous disease. Common, worldwide infection
Equine herpesvirus 3
Horses
Mild, venereal infection characterized by lesions on the external genitalia of mares and stallions
Equine herpesvirus 4
Horses
Cause of equine rhinopneumonitis in young horses and sporadic cause of abortion. Common, worldwide infection
Porcine herpesvirus 1
Pigs
Cause of Aujeszky’s disease in pigs, characterized by high mortality in piglets, weight loss and respiratory disease in fatteners and reproductive disorders in sows and boars. Transmission to other farm species may occur, associated with a fatal neurological disease (pseudorabies)
Porcine herpesvirus 2 (pig cytomegalovirus)
Pigs
Associated with upper respiratory tract infection in young pigs (inclusion body rhinitis) and generalized infection of foetuses. Widespread distribution but infection inapparent in herds where enzootic
Canine herpesvirus
Dogs
Uncommon cause of disease, associated with fatal, generalized infection in susceptible neonates
Feline herpesvirus
Cats
Common infection with worldwide distribution. Cause of feline viral rhinotracheitis, an acute, upper respiratory tract disease of young cats
Anatid herpesvirus 1
Ducks (duck plague), geese, swans
Highly contagious, acute disease characterized by ocular and nasal discharge, diarrhoea and high mortality. Worldwide distribution
Gallid herpesvirus 1
Chickens
Cause of infectious laryngotracheitis, an acute, upper respiratory tract disease. Worldwide distribution. Significant mortality associated with high-virulence strains of virus
Gallid herpesvirus 2 (Marek’s disease virus)
Chickens
Marek’s disease is an economically important, lymphoproliferative disease of chickens characterized by leg and wing paralysis in 12–24-week-old birds. Infection is common and worldwide
Pigeon herpesvirus 1
Pigeons
Conjunctivitis and upper respiratory tract infection of young birds. Asymptomatic, latent infection in adult birds. Worldwide distribution
Psittacid herpesvirus 1
Psittacine species
Cause of Pacheco’s disease. Fatal, generalized infection associated with stress
Infectious bovine rhinotracheitis/infectious pustular vulvovaginitis
Diagnosis
Malignant catarrhal fever
Pathogenesis
Diagnosis
Bovine herpes mammillitis and pseudo-lumpy skin disease
Diagnosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree