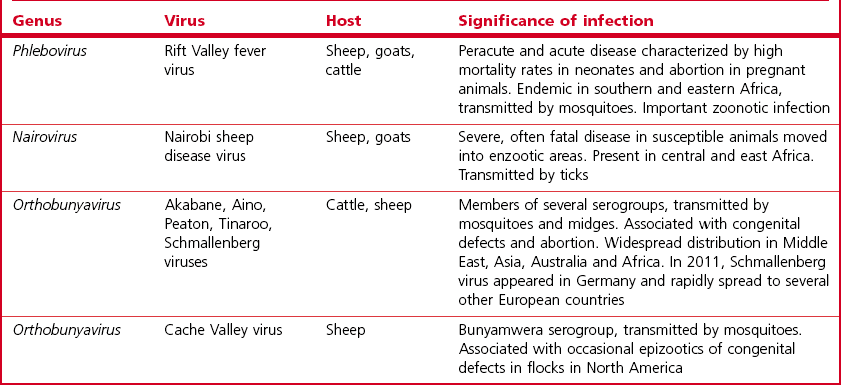
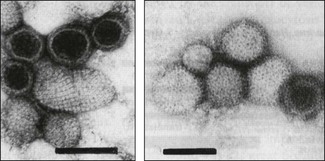
Chapter 64 This family is one of the largest, with more than 300 member species. Bunyaviruses derive their name from Bunyamwera, the location in Uganda where the type species Bunyamwera virus was first isolated. The viruses are spherical, 80 to 120 nm in diameter and enveloped. The envelope is studded with projecting glycoprotein peplomers and encloses three circular, helical nucleocapsids (Fig. 64.1). The genome is made up of three circular, negative-sense (ambisense in the case of members of the genera Phlebovirus and Tospovirus) single-stranded RNA segments, which differ in size and are designated small (S), medium (M) and large (L). Four structural proteins: a large (L) transcriptase protein, a nucleocapsid (N) protein and two external glycoproteins (Gn and Gc) are described. Nonstructural (NS) proteins are coded for by the S segment of RNA (designated NSs) and by the M segment (designated NSm). Genetic reassortment occurs between closely related viruses. Replication occurs in the cytoplasm of host cells. The envelope is acquired by budding into the Golgi cisternae. Transport through the cytoplasm in vesicles and release at the cell surface by exocytosis completes the replication cycle. Figure 64.1 Negatively stained electron micrographs of; (left) Hantaan virus virions showing the pattern of peplomer placement in squares that is characteristic of all hantaviruses, (right) Rift Valley fever virus virions showing the delicate peplomer fringe. Bars represent 100 nm. Reprinted with permission: Veterinary Virology Third Edition (1999). Murphy et al., Academic Press. Family Bunyaviridae. Page 471. Members of the Bunyaviridae family are grouped into five genera; Orthobunyavirus, Phlebovirus, Nairovirus, Hantavirus and Tospovirus. Within each genus viruses are placed into serogroups on the basis of serological testing and antigenic relatedness. More than 40 serogroups/species have been described in the genus Orthobunyavirus. A relatively small number of viruses in the family are significant pathogens of vertebrates and are contained in the genera Orthobunyavirus, Phlebovirus, Nairovirus and Hantavirus while the genus Tospovirus contains viruses of plants (Fig. 64.2). The viruses are sensitive to heat, acid pH, lipid solvents, detergents and many disinfectants. In all genera except Hantavirus the viruses are arboviruses and maintained in nature in complex life cycles involving replication in arthropod vectors and vertebrate hosts (Table 64.1). Infection of mammalian cells results in cytolysis while infection of invertebrate cells is non-cytolytic and persistent. Mosquitoes form the largest, most important group of vector species but ticks, sandflies and biting midges are important for certain virus species. Transovarial and venereal transmission of virus has been shown to occur in vector species. Vertebrate hosts infected during blood feeding by the vector serve to amplify the amount of virus in circulation and pass on infection to susceptible arthropods during a viraemic period of variable intensity and duration. Virus species tend to display a narrow host range, particularly in their arthropod vectors. In contrast, hantaviruses are maintained in nature by chronic, nonpathogenic infections of rodents. Hantavirus infections established in susceptible mammalian cell lines are typically non-cytolytic and persistent. Virus is shed in urine, faeces and saliva facilitating transmission between rodent hosts by aerosol and biting. Each hantavirus species is associated with a particular rodent species. Outbreaks of disease tend to occur in eastern and southern Africa at irregular intervals of five to 15 years or more. In recent years Rift Valley fever virus (RVFV) has spread to the Arabian Peninsula (Al-Afaleq et al. 2003). Outbreaks are associated with abnormally heavy rains and an explosive increase in vector populations. Transovarial transmission of RVFV occurs in Aedes species and the virus survives for very long periods in mosquito eggs dormant in the soil during dry periods. In an epidemic, RVFV is amplified in both domestic and wild ruminant species and can be transmitted by many species of mosquito which become very numerous after heavy rains. Infected mosquitoes frequently transmit the infection to humans during such epidemics. A high level of viraemia occurs in infected ruminants for three to five days after infection and the blood and tissues of such animals are infectious. Abattoir workers and veterinarians are at particular risk.
Bunyaviridae
Clinical infections
Rift valley fever
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree