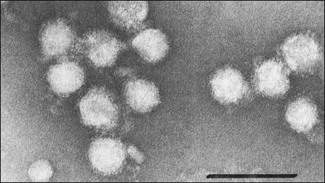
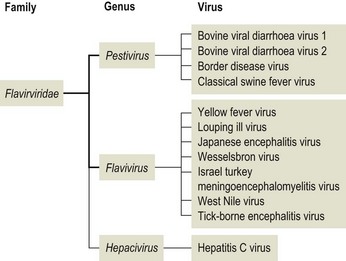
Chapter 57 The name of the family Flaviviridae is derived from the Latin word flavus meaning yellow and referring to the virus of yellow fever, which is a type species within the family. The viruses are spherical, 40–60 nm in diameter with an icosahedral capsid and a tightly adherent envelope containing two or three virus-encoded proteins depending on the genus (Fig. 57.1). The family is made up of three genera containing over 60 species: Flavivirus, Pestivirus and Hepacivirus (Fig. 57.2). The genera are antigenically unrelated but serological cross-reactivity occurs between members within the genera Flavivirus and Pestivirus. The Flavivirus genus is the largest containing about 50 species arranged into several serologically defined groups. A fourth genus, Pegivirus, has been proposed for a group of viruses isolated from primates (Pegivirus A, also known as hepatitis G virus) and from fruit bats (Pegivirus B). Figure 57.1 Negative stain electron micrograph of central European tick-borne encephalitis virus. The bar represents 100 nm. Reprinted with permission: Veterinary Virology Third Edition (1999). Murphy et al., Academic Press. Page 558. Figure 57.2 Classification of members of the family Flaviviridae with emphasis on species which affect domestic animals. The majority of members of the genus Flavivirus are arboviruses requiring either a mosquito or a tick vector (Table 57.1). Two major groups of mosquito-borne flaviviruses are distinguished on the basis of ecology and disease presentation in man. The encephalitic group includes Japanese encephalitis virus, West Nile virus, Murray Valley encephalitis virus and St Louis encephalitis virus, which infect birds as the natural vertebrate hosts and Culex species of mosquitoes as the primary vectors. The viscerotropic group includes yellow fever virus and dengue fever virus, which infect lower primates as the vertebrate hosts and Aedes species of mosquitoes as the principal vectors. About 30 members of the Flavivirus genus are known to be associated with disease in man including yellow fever, dengue, Japanese encephalitis, St Louis encephalitis, West Nile encephalitis and tick-borne encephalitis. Japanese encephalitis virus, St Louis encephalitis virus and West Nile virus are members of the same serogroup (Japanese encephalitis virus group). A number of members of the genus Flavivirus, including louping ill, Japanese encephalitis, West Nile and Wesselsbron viruses, cause disease in domestic animals. Table 57.1 Flaviviruses and pestiviruses of animals The four members of the Pestivirus genus, bovine viral diarrhoea virus (BVDV) 1 and 2, border disease virus (BDV) and classical swine fever (hog cholera) virus (CSFV), infect ruminants and pigs. They are antigenically diverse, antigenically cross-reactive and display an overlapping host spectrum. Bovine viral diarrhoea virus and border disease virus can cross-infect other ruminants and swine. Genetic studies based on comparison of the immunodominant major envelope glycoprotein E2 (gp55) indicate six genotypes within the genus (van Rijn et al. 1997); classical swine fever virus isolates, border disease virus isolates, bovine viral diarrhoea virus isolates predominantly from cattle (classical BVDV isolates), bovine viral diarrhoea virus isolates from sheep, swine and cattle (atypical BVDV) isolates, deer pestivirus isolate and giraffe pestivirus isolate. A novel pestivirus genotype has been identified in pronghorn antelope (Vilcek et al. 2005). The analysis of pestivirus genetic sequences suggests three main branches in the pestivirus ‘family tree’; the first branch includes BVDV 1 and 2, the second branch includes CSFV, BDV and pestiviruses from giraffe and reindeer, the third branch has a single member from a pronghorn antelope (Ridpath 2003). Liu et al. (2009) have proposed nine species of pestivirus. Pestivirus infections may be inapparent, acute or persistent and are important economically worldwide. Hepatitis C virus is currently the only member of the Hepacigenus and is an important cause of hepatitis in humans. Infection with bovine viral diarrhoea virus (BVDV) is common in cattle populations worldwide. Successful eradication schemes have been carried out in Scandinavia. The virus is responsible for acute disease (bovine viral diarrhoea) and a chronic syndrome associated with persistent infection (mucosal disease). Isolates of BVDV can be segregated into two genotypes now considered separate species, BVDV 1 (classical BVDV isolates) and BVDV 2 (atypical BVDV isolates), on the basis of comparison of the conserved 5′ untranslated region of the viral genome. Isolates of the two viruses can be further subdivided into a and b subgenotypes (Ridpath 2003) and in the case of BVDV 1 several additional subgenotypes (Vilcek et al. 2001). Both BVDV 1 and BVDV 2 can exist as one of two biotypes based on their activity in cell cultures: cytopathic or non-cytopthic. Non-cytopathic isolates circulate widely in cattle populations. Cytopathic isolates arise from noncytopathic BVDV as a result of recombination events that include the insertion of host RNA, the duplication of viral RNA sequences or mutations in the NS2-3 gene (Meyers et al. 1996, Kummerer et al. 2000). In some cases the cytopathic mutant results from recombination between the resident non-cytopathic virus and a superinfecting heterotypic cytopathic virus, such as has occurred in vaccine-associated outbreaks. Bovine viral diarrhoea virus 1 and BVDV 2 produce similar clinical syndromes in cattle. However, only non-cytopathic BVDV 2 isolates have been associated with thrombocytopenia and a haemorrhagic syndrome, first described in North America and now referred to as severe acute BVD (Rebuhn et al. 1989). The BVDV isolates used in vaccines and diagnostic tests have traditionally been BVDV 1. Animals exposed to BVDV for the first time transiently shed the virus in the early stages of infection and may transmit infection to other animals. Chronic shedding of virus in semen has been reported (Voges et al. 1998). Of far greater importance is the role of persistently infected animals, which shed the virus in all excretions and secretions and are efficient transmitters of the infection. Persistent infections are produced following foetal infection before day 120 gestation with non-cytopathic strains. Approximately 1% of animals in an infected population are persistently infected and viraemic while from 60% to 85% are antibody-positive (Houe 1999). Persistently infected cows may breed and will always transmit the virus to the calf. The familial occurrence of persistent infection is reasonably common. The presence of persistently infected animals in a herd results in ongoing exposure to the virus and a high level of herd immunity. Typically more than 80% of animals in infected herds are serologically positive. Transplacental spread to the foetus will occur in susceptible pregnant animals. The outcome of foetal infection is highly dependent on the age of the foetus. During the first 30 days of gestation early embryonic death and infertility may follow infection. Infection during the first and second trimesters may result in abortion, mummification or congenital abnormalities such as cerebellar hypoplasia. Foetuses infected during the last trimester are able to mount an active immune response and are usually normal at birth. Foetal infection occurring up to about day 120 may result in the birth of a persistently infected calf. The developing immune system of the foetus does not recognize the virus as foreign but comes to regard viral antigens as self-antigens. As a result of this immunotolerance, specific antibody is not produced and the virus is able to persist for the lifetime of the animal. At some stage after birth, usually betwen six to 24 months of age, an antigenically homologous cytopathic biotype of virus arises as a result of a genetic alteration in the virus. This event is responsible for the subsequent development of mucosal disease. Cytopathic isolates demonstrate a pronounced tropism for gut-associated lymphoid tissue and continually produce an 80-kDa non-structural protein known as NS3 (p80), due to cleavage of the NS2-3 gene product. The role of NS3 in the pathogenesis of mucosal disease is unclear but is correlated with the development of disease. Non-structural 3 protein is present in leukocytes and lymphoid tissue in cattle persistently infected with non-cytopathic virus (Kameyama et al. 2008) and may account for signs such as poor development and reduced immunocompetence reported in some persistently infected animals. The virus has a necrotizing effect on epithelial tissues of the gastrointestinal tract, integument and respiratory tract. • Virus isolation is possible in a wide range of cell cultures; primary bovine kidney, turbinate and testis cells are highly sensitive. The harvesting of the buffy coat from whole blood provides a useful specimen in the live animal. Suitable specimens from post mortem cases include spleen, liver, kidney, lymph node and sections of the gastro-intestinal tract containing lesions. Two samples taken three weeks apart should be analysed to confirm persistent infection in an animal. Cell lines and foetal calf serum, used as a cell culture medium supplement, must be screened for the presence of BVDV before attempting isolation of the virus (Bolin et al. 1991). The foetal calf serum should also be free from BVDV-specific antibodies. Cytopathic BVDV isolates will produce foci of lysed cells in the cell monolayer while non-cytopathic virus can be detected using fluorochrome or enzyme-labelled BVDV-specific antibodies. A microtitre virus isolation method using sera, the immunoperoxidase monolayer assay (IPMA), for whole herd screening for PI cattle has been developed (Saliki et al. 1997). It has been reported that some PI cattle can be IPMA-negative on serum but virus-isolation-positive on buffy coat (Grooms et al. 2001). • Viral antigen can be detected directly by immunofluorescence on frozen sections or buffy coat smears. Immunohistochemical staining of skin sections (‘ear notch’ test) has been shown to be reliable and to correlate well with findings from blood testing (Thür et al. 1996, Njaa et al. 2000). Several antigen capture ELISAs have been developed for rapid detection of BVDV antigens extracted from tissues or blood leukocytes (Fenton et al. 1991, Shannon et al. 1991) and commercial kit sets are available. These assays are usually based on the use of monoclonal antibodies that recognize the antigenically conserved non-structural protein NS2-3 and therefore should detect most if not all BVDV strains. • Dot blot, in situ hybridization and RT-PCR techniques for the detection of viral RNA have been described (Roberts et al. 1991, Sandvik et al. 1997, McGoldrick et al. 1999, Letellier & Kerkhofs 2003) as well as a multiplex PCR for the detection and differentiation of BVDV 1 and BVDV 2 (Gilbert et al. 1999). Reverse transcription-PCR is sufficiently sensitive for the detection of viral nucleic acid in pooled samples such as bulk milk (Radwan et al. 1995, Drew et al. 1999) and serum (Weinstock et al. 2001). This is a particularly useful approach where the herd in question has been vaccinated and antibody testing is not appropriate. It is possible to discriminate between BVDV-1 and -2 using separate sets of primers and probes (Letellier & Kerkhofs 2003). • Virus neutralization and ELISA are the preferred methods for the detection of antibodies to BVDV (Edwards 1990). The virus neutralization test detects antibodies to the viral glycoproteins, particularly E2 (gp55). Although these antibodies are capable of cross-reacting with several strains of BVDV, it is important to use a challenge virus antigenically similar to the field virus circulating in the test population. The tissue culture cells used can also influence the apparent titre of a test serum (Saliki & Dubovi 2004). ELISA kits for detection of BVDV-specific antibodies are available commercially. The collection of paired serum samples and the detection of a fourfold increase in antibody titre are necessary to detect recent infection. The systematic serological testing of bulk milk or pooled blood samples from herds is an important part of identifying herds with PI animals and as such is also a key step in national eradication programmes. In order to confirm the removal of all PI animals from a herd, unvaccinated animals between eight to 12 months should be tested for antibodies. These animals are free of maternal antibody and have been at risk of infection for some time. The use of antibody testing as a screening test for PI animals is not recommended where BVDV vaccines are being used as PI cattle can have neutralizing antibody titres to the vaccine strain of the virus. The NS3 protein, alone or in combination with other BVDV proteins, is frequently used as the basis of commercial ELISAs because it is highly conserved among the pestiviruses. Recent studies have shown that NS3-specific antibody levels in serum and milk are low or undetectable following vaccination with an inactivated BVDV vaccine. As a result it is possible to monitor infection in vaccinated herds using a suitable combination of inactivated vaccine and NS3 antibody test (Makoschey et al. 2007, Kuijk et al. 2008).
Flaviviridae
Virus
Host species
Disease
Bovine viral diarrhoea virus 1 and 2
Cattle (sheep, pigs)
Causes inapparent infection, acute disease (bovine viral diarrhoea) and sporadic fatal infection (mucosal disease). Infection of pregnant animals may result in abortion, congenital defects or persistent infection (immunotolerance)
Border disease virus
Sheep
Important infection of pregnant ewes and cause of abortion or congenital abnormalities (hairy shaker lambs)
Classical swine fever (hog cholera) virus
Pigs
Economically significant disease. Highly contagious, generalized infection that is frequently fatal. Nervous signs, abortion and congenital tremor are features of the disease
Louping ill virus
Red grouse, sheep, cattle, horses and humans
Present in specific regions of Europe. Transmitted by the tick Ixodes ricinus. Produces encephalitis in sheep
Japanese encephalitis
Water birds, pigs, horses and humans
Widespread distribution in Asia. Transmitted by mosquitoes. Water birds are reservoir host. Infection in pigs is associated with abortion and neonatal mortality. May cause nervous disease in horses
West Nile virus
Birds, humans and horses
Transmitted by mosquitoes; birds are the natural hosts. Sporadic cause of serious CNS disease in humans and horses
Wesselsbron virus
Sheep
Occurs in parts of sub-Saharan Africa. Causes generalized infection, hepatitis and abortion. Transmitted by mosquitoes
Israel turkey meningoencephalitis virus
Turkeys
Outbreaks of progressive paresis and paralysis in turkeys in Israel and South Africa. Mosquito-borne virus
Bovine viral diarrhoea
Pathogenesis
Diagnosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Flaviviridae
Only gold members can continue reading. Log In or Register to continue
