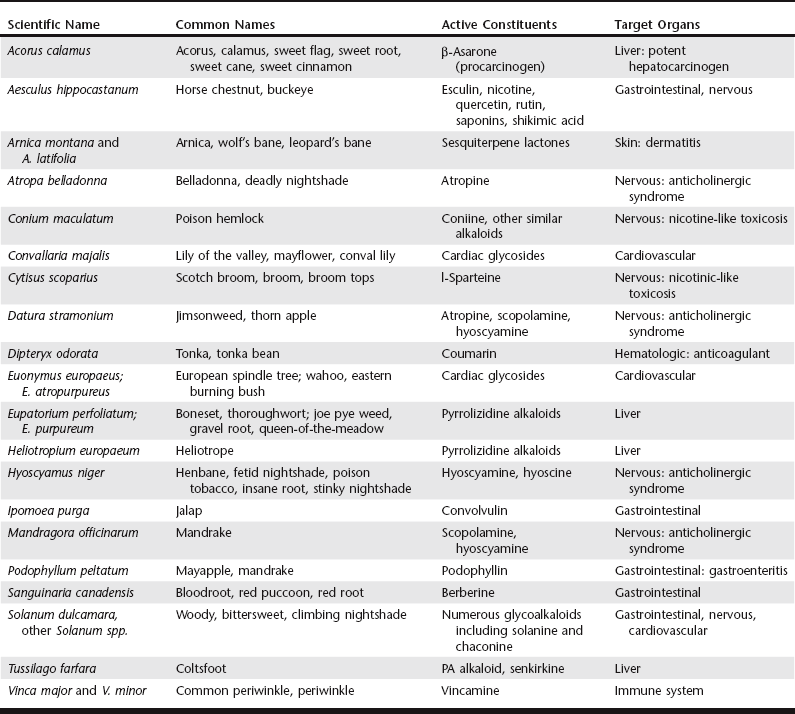
Chapter 29 There are a number of scenarios in which animals may experience an adverse reaction to or toxicosis from an herbal preparation. It is worth noting that in several reports the incidence of animal intoxication from an herb, herbal preparation, or dietary supplement seems to parallel its popularity (Ooms, Khan, and Means, 2001; Gwaltney-Brant, Albretsen, and Khan, 2000). The following list provides situations in which toxicosis may occur: 1. The components of a supplement are correctly identified and the preparation contains a known toxicant. For example, the dried rootstocks of Aconitum spp. contain several constituents that are acutely cardiotoxic (Lin, Chan, and Deng, 2005). Pennyroyal oil containing the putative toxin pulegone was responsible for the death of a dog after dermal application to control fleas (Sudekum et al, 1992). 2. The intoxication may be chronic rather than acute as in the case of pyrrolizidine alkaloids, which are found in many plant species and when ingested over time cause severe liver disease (Prakash et al, 1999; Stedman, 2002). 3. Errors may be made when preparing a remedy. For example, human illness was caused when an anise seed preparation was contaminated with the highly toxic Conium maculatum (poison hemlock) seed (deSmet, 1991). An outbreak of renal interstitial fibrosis in women taking Chinese herbs for weight loss was attributed to the use of Aristolochia fangchi instead of Stephania tetrandra in imported powdered extracts (Vanherweghem, 1998). 4. Herbal preparations may unintentionally contain contaminants or intentionally contain chemical adulterants. Many Chinese herbal patent medicines contain drugs such as nonsteroidal antiinflammatory drugs (NSAIDs) or sedatives (Ko, 1998). Also, heavy metal and pesticide contamination has been reported (Ernst, 2002b; Saper et al, 2004; Harris et al 2011). Salmonellosis has been reported in humans taking rattlesnake capsules contaminated with Salmonella arizonae (Fleischman, Haake, and Lovett, 1989). 5. The active constituents in herbal preparations can interact with other concurrently administered drugs, resulting in adverse interactions. For example, buckthorn bark and berries taken chronically can increase the loss of potassium, thus potentiating the action of cardiac glycosides and antiarrhythmic agents (DerMarderosian, 2001). Potassium loss may be exacerbated by simultaneous use of thiazide diuretics, corticosteroids, and licorice root (DerMarderosian, 2001). In addition, active constituents in herbal preparations can induce liver-metabolizing enzymes, which can alter the metabolism and kinetics of coadministered conventional drugs. For example, eucalyptus oil induces liver enzyme activity (DerMarderosian, 2001). This scenario of adverse pharmacologic interactions may occur when the owner does not inform the veterinarian of the intentional use of herbal preparations. 6. Finally, pets may consume improperly stored remedies, resulting in ingestion of a large quantity of a product. There are many different ethnic traditions of herbal medicine use, with many plants having roles in these remedies. The following broad classes of active chemical constituents occur in plants: volatile oils, resins, alkaloids, polysaccharides, phenols, glycosides, and fixed oils (Hung, Lewin, and Howland, 1998). Volatile oils are odorous plant ingredients (e.g., catnip, garlic, citrus). Resins are complex chemical mixtures that can be strong gastrointestinal irritants. Alkaloids are a heterogeneous group of alkaline, organic, and nitrogenous compounds. Glycosides are sugar esters containing a sugar (glycol) and a nonsugar (aglycon). In some cases the glycosides are not toxic. However, hydrolysis of the glycosides after ingestion can release toxic aglycons. Fixed oils are esters of long-chain fatty acids and alcohols. Herbs containing fixed oils are often used as emollients, demulcents, and bases for other agents; in general these are the least toxic of the plant constituents. There is a misperception that preparations from plants are inherently safe because they occur in nature compared with synthesized chemicals. However, many plant-derived chemicals are biologically active and therefore potentially toxic. Concentrated extracts of a number of herbs have proven to be toxic even if the entire plant may be used with relative safety. Although green tea is consumed by many people with apparent safety, an extract of green tea marketed in Europe caused a significant number of adverse hepatic events, including fulminant hepatitis. The extract was withdrawn from the market (Gloro et al, 2005). Some of the most commonly encountered herbals are discussed in the following paragraphs; others are listed in Table 29-1. The dried young branches of ephedra (Ephedra spp.) have been used for their stimulating and vasoactive effects. In addition, ephedra has been used in several products promoted for weight loss. The plant constituents responsible for biologic activity are the sympathomimetic alkaloids ephedrine and pseudoephedrine. A case series involving intoxication of dogs following ingestion of a weight-loss product containing guarana (caffeine) and ma huang (ephedrine) has been reported (Ooms, Khan, and Means, 2001). Estimated doses of the respective plants associated with adverse effects were 4.4 to 296.2 mg/kg for guarana and 1.3 to 88.9 mg/kg for ma huang. Symptomatology included hyperactivity, tremors, seizures, behavioral changes, emesis, tachycardia, and hyperthermia. Ingestion was associated with mortality in 17% of the cases. North American species of ephedra (also called Mormon tea) have not been shown to contain the sympathomimetic alkaloids. Citrus aurantium (“bitter orange” or “Seville orange”) has appeared in many products labeled as “ephedrine-free” and is also combined with caffeine and/or guarana. The primary active components of C. aurantium are synephrine (structurally similar to epinephrine), octopamine (structurally similar to norepinephrine), and N-methyltyramine. The overall effect is that of stimulation (Fugh-Berman and Myers, 2004). Studies in humans have shown that bitter orange–containing preparations cause tachycardia and increases in systolic and diastolic pressure (Haller, Benowitz, and Jacob, 2005). Signs of intoxication are similar to those seen with ephedra. Guarana is the dried paste made from the crushed seeds of Paullinia cupana or P. sorbilis, a fast-growing shrub native to South America. The primary active component in the plant is caffeine, with concentrations that range from 3% to 5% compared with 1% to 2% for coffee beans. Currently the most common forms of guarana include syrups, extracts, and distillates used as flavoring agents and as a source of caffeine for the soft-drink industry. More recently it has been added to weight-loss formulations in combination with ephedra. Oral lethal doses of caffeine in dogs and cats range from 110 to 200 mg/kg of body weight and 80 to 150 mg/kg of body weight, respectively (Carson, 2001; also see Ephedra earlier in the chapter for a discussion of a case series involving dogs ingesting a product containing guarana and ephedra; Ooms, Khan, and Means, 2001). Kratom usually refers to the leaves of Mitragyna speciosa, a plant indigenous to Southeast Asia. Kratom, or Krypton, has been used traditionally for pain, depression, and anxiety. There is some recent use for treating symptoms associated with opiate withdrawal. The leaves contain several active components including mitragynine and 7-hydroxymitragynine (Horie et al, 2005). While structurally similar to yohimbine, mitragynine acts as a mu opioid receptor partial agonist. The relatively minor component, indole alkaloid 7-hydroxymitragynine, is reported to be more potent than morphine. Kratom is a controlled substance in some countries, but not the United States (Babu et al, 2008). There are case reports in the literature describing adverse effects in humans but as yet little clinical information for animals (Kapp et al, 2011). Noni juice is derived from Morinda citrifolia, sometimes called “starvation fruit” due to a taste unappealing to humans. Traditionally, noni juice (also called Ba Ji’Tian, Indian Mulberry, or Wild Pine) has been used for a variety of conditions ranging from asthma to smallpox, premenstrual syndrome, and leprosy but is not well studied for any of those. The herbal has a high potassium content that may predispose to interactions with potassium-sparing diuretics and certain antihypertensive medications. Xeronine and proxeronine are components mentioned in advertising, but at this time they have not been chemically identified or studied medically. Although there are several reports of hepatic damage in humans, there are insufficient data at this time to assess safety and efficacy in animals (Yue et al, 2011; Millonig et al, 2005; Stadlbauer et al, 2008). Some of the available salicylate-containing plants and oils are listed in Tables 29-2 and 29-3, respectively. TABLE 29-2
Herbal Hazards
Intoxication Scenarios
Active Herbal Constituents
Toxicity of Specific Herbs or Other Natural Products
Ephedra or Ma Huang
Guarana
Kratom
Noni Juice
Salicylate-Containing Preparations
Common Name
Latin Name
Salicylate Content
White willow
Salix alba
Variable
Sweet birch
Betula lenta
Variable
White birch
Betula pendula
Variable
Meadowsweet
Filipendula ulmaria
Variable ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Herbal Hazards
Only gold members can continue reading. Log In or Register to continue