Chapter 46 Hepatobiliary Cytoprotective Agents
Natural Hepatobiliary Defense Mechanisms
Hepatobiliary cells also respond to toxic signals by initiating intracellular prosurvival biochemical pathways. These pathways, which protect against necrotic and apoptotic cell death, are controlled by hormones, such as glucagon, and growth factors, such as hepatocyte growth factor, and work through the modulation of survival kinases, including phosphoinositol-3-kinase (PI3K) and Akt.1
Hepatoprotective Agents
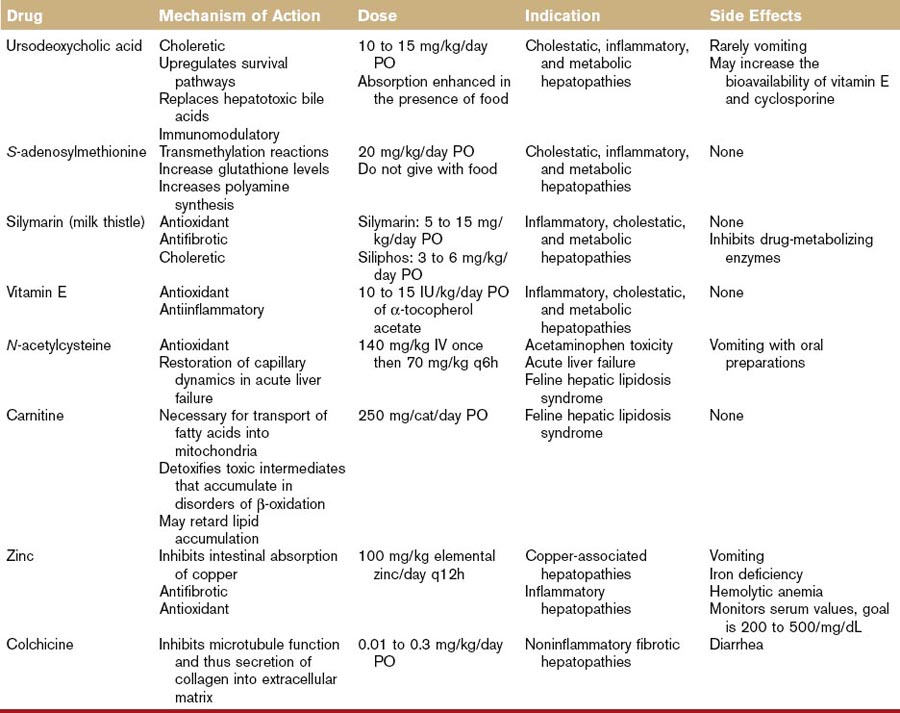
A summary of the hepatoprotective agents discussed in the following section can be found in Table 46-1.
Ursodeoxycholate
Chinese black bear bile has been used for its hepatobiliary healing power for centuries. The major bile acid in this bear is ursodeoxycholate (UDCA). Bile acids, a family of molecules synthesized exclusively in the liver, are formed when a hydroxyl group (OH) is added to cholesterol’s steroid nucleus. The simplest bile acids are thus the di-OH bile acids, chenodeoxycholate and UDCA. Additional hydroxylation creates the tri-OH bile acids in the cholate group. Bile acids are conjugated to either taurine or glycine in the liver. In cats and dogs, the tri-OH bile acid, taurocholate, is the major circulating bile acid. Some bile acids, unlike UDCA, are hepatotoxic.2 The structural basis for the differential cytotoxicity of bile acids is not fully understood but is roughly correlated with their degree of hydrophobicity. Toxic bile acids damage hepatocytes by disrupting biologic membranes and stimulating apoptosis.2
UDCA’s cytoprotective effect is associated with several factors.3 First, UDCA can replace more hydrophobic hepatotoxic bile acids from the circulating bile acid pool. This effect is of limited value in dogs and cats in which the major circulating bile acid is the relatively nontoxic, taurocholate. A major cytoprotective action of UDCA lies in is its ability to inhibit apoptosis. Mitochondria are key regulators of apoptotic pathways and UDCA can stabilize mitochondrial membrane function, increase mitochondrial stores of glutathione, and prevent the mitochondrial-mediated generation of free radicals, all of which contribute to mitochondrial instability and the initiation of apoptosis. UDCA’s antiapoptotic action also involves stimulation of cellular survival signaling through activation of protein (Akt, mitogen-activated kinases) and lipid kinases (PI3K). UDCA is a choleretic agent and this action promotes the excretion of potentially toxic endogenous metabolites retained during cholestasis. UDCA induces choleresis by (a) direct stimulation of a bicarbonate-rich bile flow in the bile ducts (followed by the osmotic movement of water) and (b) increasing the membrane expression of transport proteins necessary to generate bile flow. Emerging evidence suggests that UDCA also works as a biologic response modifier of the glucocorticoid receptor. UDCA can activate the glucocorticoid receptor by interacting with a distinct region of the receptor (not the same binding site as cortisol), inducing nuclear translocation of the glucocorticoid receptor, and suppressing transcription of inflammatory mediators.
There is little information in the literature on the use or efficacy of UDCA in small animals. The recommended dose (10 to 15 mg/kg/day PO), has been extrapolated from human medicine. One study each in the cat and the dog shows that oral administration of this dose results in the appearance of UDCA in the serum.4,5 In the dog case report, this dose was associated with biochemical and clinical improvement of chronic hepatitis.
UDCA is well tolerated. Diarrhea and vomiting may occur rarely. Extensive toxicologic studies performed in healthy dogs did not reveal any serious side effects. No adverse effects were noted in normal cats treated with 10 mg/kg/day PO for 3 months or with 15 mg/kg/day PO for 8 weeks.4,6 UDCA can increase the bioavailability of cyclosporine and vitamin E.
The effect of UDCA on serum total bile acid concentrations has been investigated in normal dogs and cats. In cats given 15 mg/kg/day, pre- and postprandial serum bile acid concentrations increase but are not out of the normal range.4 In normal dogs, one study showed that 15 mg/kg/day UDCA increased postprandial serum bile acids in only one of 16 dogs whereas another study using the same dose showed that six of 14 dogs developed increased serum total bile acids 1 to 6 hours after oral dosing.7,8 The effect of UDCA administration on serum bile acid profiles in animals with hepatic disease has not been reported.
S-Adenosylmethionine
The liver is the major site of S-adenosylmethionine (SAMe) synthesis and degradation. Methionine is actively transported into the liver and converted to SAMe by the enzyme, methionine adenosyltransferase. The activity of methionine adenosyltransferase is impaired in experimental models of liver injury in laboratory rodent and in human patients with alcoholic cirrhosis. Thus, in the setting of severe liver disease, SAMe becomes a conditionally essential nutrient.9,10
In the liver there are three metabolic pathways for SAMe metabolism: (a) transmethylation, (b) transsulfuration, and (c) aminopropylation, and all three are implicated in the compound’s hepatoprotective effects.9,10 In transmethylation reactions, SAMe donates a methyl group to a large number of molecules, including proteins, DNA, and membrane phospholipids. Through methylation of the latter two, SAMe can decrease the expression of inflammatory cytokines and stabilize mitochondrial membranes. Once SAMe has donated its methyl group, it is converted to S-adenosylhomocysteine. In the transsulfuration pathway, S-adenosylhomocysteine can be converted by a series of enzymatic steps to cysteine. Because the availability of cysteine is the rate-limiting step in the synthesis of glutathione, SAMe administration can increase hepatic glutathione levels in normal dogs and cats.11,12 It also ameliorates acetaminophen-induced red blood cell and hepatic damage in cats and dogs, respectively.13,14 In the third major pathway, SAMe can be decarboxylated to polyamines that can stimulate protein synthesis. Methyladenosine, an intermediate in this pathway, is antiapoptotic in hepatocytes.
Two stable salts of SAMe are available for oral administration.15 The 1,4-butanedisulfonate salt is marketed for veterinary use as Denosyl-SD4 (Nutramax Laboratories, Inc.) and the tosylate salt is marketed as Zentonil (EVSCO Pharmaceutical). The dose is 20 mg/kg/day. SAMe tablets must be enteric coated and stored in a blister pack. The tablets should not be split or crushed. Because food interferes with absorption, SAMe should be given in the fasting state. SAMe has low oral bioavailability (approximately 3%) because of a significant hepatic first pass effect and rapid metabolism within the liver. SAMe crosses the blood–brain barrier16 and there is a significant body of literature to support an antidepressant effect in humans.17 SAMe is rapidly metabolized intracellularly, but that portion not metabolized undergoes renal and fecal excretion. Limited pharmacokinetic studies in dogs show that peak plasma concentrations occur within 1 to 4 hours.12 No side effects have been reported in human trials and the LD50 (median lethal dose for 50% of test subjects) in laboratory rats is greater than 4650 mg/kg/day. Cats given four times the recommended dose for 3 months had no adverse effects.11
In a study of prednisone-treated dogs, SAMe did not prevent the development of hepatic vacuolar changes or the induction of serum hepatic enzyme activity.12 In this study, concurrent prednisone use did not adversely affect the pharmacokinetics or pharmacodynamics of SAMe administration.
In human clinical trials, SAMe has shown beneficial effects in alcoholic and cholestatic liver disorders.18,19 The therapeutic potential of SAMe in veterinary medicine is unknown as limited clinical studies have been published. Because glutathione levels are decreased in some animals with hepatic disease,20,21 particularly cats with hepatic lipidosis, SAMe supplementation may be beneficial. SAMe may also be useful as adjunctive therapy in dogs and cats with inflammatory liver disease. This author often uses SAMe in combination with corticosteroids and/or ursodeoxycholate. There is some evidence that the concurrent use of SAMe and ursodeoxycholate may be synergistic.19 Typically, animals are given a 3-month trial of SAMe to evaluate whether addition to the therapeutic protocol provides symptomatic or biochemical improvement. Because SAMe crosses the blood–brain barrier and there is a considerable body of evidence to suggest that SAMe is effective in the treatment of depression in humans, the possibility of “mood elevating” effects in symptomatic control of hepatopathies cannot be overlooked. SAMe therapy may be useful in the recovery phase of hepatotoxic drug reactions.22
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree