37 Hepatic insufficiency
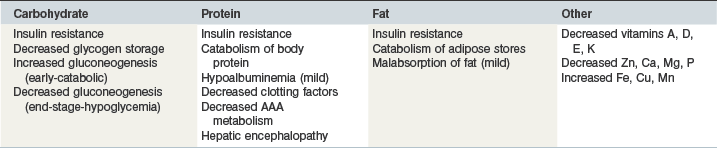
The equine liver constitutes approximately 1% of bodyweight in the horse (Sisson 1975) and plays a key role in many aspects of equine nutrition including synthetic, storage, processing and digestive functions pertaining to all major and many minor nutrients. Failure of the liver to accomplish its many roles may lead to disturbance of nutrient availability and homeostasis potentially leading to several clinical nutritional problems including those listed in Table 37-1.
There is no reason to suspect that the dietary requirements of horses suffering from compensated liver disease differ from normal horses and specific dietary change is unnecessary in such cases other than perhaps screening for potential dietary hepatotoxins. However if compromise of normal hepatic functions is suspected on the basis of clinical (e.g. weight loss, photodermatitis) or clinicopathological (e.g., increased serum bile acid concentration, hypoalbuminemia) evidence then dietary manipulations should be considered. Dietary management has been shown to significantly improve morbidity and mortality in human patients with liver failure (Alberino et al 2001, Manguso et al 2005) and might be expected to be equally important in equine cases. Unfortunately there is a scarcity of good quality data to help provide evidence-based nutritional guidelines for hepatic insufficiency in horses. Extrapolation of information derived from other species is a poor substitute for specific equine evidence but might be considered and applied cautiously in the absence of the latter.
Metabolic consequences of hepatic insufficiency
Protein-energy malnutrition
Relative deficiency in both dietary protein and energy (protein-energy malnutrition) is commonly recognized in human patients with hepatic insufficiency (Caregaro et al 1996) and weight loss is also one of the most frequently observed signs in horses with hepatic failure (McGorum et al 1999, Durham et al 2003a). Several factors may lead to protein-energy malnutrition including inappetance, mild fat malabsorption and insulin resistance. Insulin resistance is a common feature of hepatic failure in humans (Arakawa et al 2004) and horses (A.E. Durham unpublished data) and reduces tissue glucose uptake and glycogen synthesis (Blei et al 1982, Petrides et al 1994). Consequent depletion of normal glycogen reserves creates a greater reliance on gluconeogenesis in the absence of constant dietary intake and this is also facilitated by an insulin resistant status (Owen et al 1983). Gluconeogenesis relies upon substrates including amino acids from protein degradation and glycerol from lipolysis leading to a general catabolic status and an abnormal reliance on body protein for energy (Swart et al 1988). It has been shown that an overnight fast in a cirrhotic patient is metabolically similar to 2 or 3 days of total starvation in normal subjects (Owen et al 1983). Ultimately functional impairment of hepatic gluconeogenesis in an end-stage failing liver may have dire consequences on plasma and tissue glucose homeostasis with hypoglycemia a probable result (West 1996).
Hepatic encephalopathy
Dietary protein has long been implicated in the pathophysiology of HE as a potential source of neurotoxic ammonia, mercaptans, oxyphenol and aromatic amino-acids (AAAs) that might generate “false neurotransmitters” (Balo & Korpassy 1932, Philips et al 1952, Schwartz et al 1954, Fischer et al 1976, Marchesini et al 1990, Holecek 2010). Recent evidence especially implicates degradation of dietary glutamine by intestinal glutaminase in the generation of systemic hyperammonemia (Romero-Gómez et al 2009). In the past the apparent association between proteins, amino acids and HE has been used to advocate the use of low-protein diets in subjects with hepatic insufficiency with or without HE (Schwartz et al 1954, Sherlock et al 1956). Intuitively however, protein-restricted diets can only reduce protein-derived neurotoxins if they provide enough energy and protein to prevent endogenous proteolysis. Furthermore, more recent human studies have indicated that normal to high protein diets are generally well tolerated (especially with concurrent lactulose therapy) and that protein restriction simply promotes catabolism of endogenous protein and might actually facilitate HE (Kearns et al 1992, Morgan et al 1995). In 1997 an evidence-based consensus guideline from the European Society of Enteral and Parenteral Nutrition (Plauth et al 1997) advocated the abandonment of protein restricted diets for humans with hepatic insufficiency with or without HE. More recently a randomized trial further demonstrated that even transient protein restriction does not benefit patients during an episode of HE (Córdoba et al 2004).
Several studies in humans have shown that patients with HE are improved when animal protein is replaced by vegetable protein in the diet (Amodio et al 2001) and it might be supposed therefore that the debated encephalopathic risks associated with dietary protein in other species may not be as significant in herbivores such as the horse. Possible reasons why vegetable protein might be less likely to provoke HE include: higher arginine content (important for conversion of ammonia to urea); lower methionine content (source of mercaptans); lower tryptophan content (source of oxyphenol and tryptamine); lower tyrosine and phenylalanine content (sources of several false neurotransmitters); and lower nitrogen assimilation due to retention by colonic flora and associated higher dietary fiber promoting higher fecal bulk and nitrogen losses (Weber et al 1985, Bianchi et al 1993, Amodio et al 2001). Interestingly the ratio of branched chain amino acids (BCAAs) to AAAs tends to be lower (i.e., theoretically less favorable) in cereals and pulses (3.28 and 2.89) compared with meat and fish (3.49 and 3.18) casting doubt on the importance of this analyte (Amodio et al 2001). However, not all studies have found vegetable protein to be significantly beneficial, although the use of vegetable protein is generally favored by dieticians in people with HE on the balance of available evidence (Blendis 1989, Amodio et al 2001). Therapeutic use of BCAA supplementation has been investigated in several human studies but the overall specific benefit remains equivocal (Als-Nielsen et al 2003, Marchesini et al 1990). Glutamine supplementation would appear inadvisable in the face of hepatic failure given its association with hyperammonemia (Romero-Gómez et al 2009).
Oral administration of lactulose, a nonabsorbable disaccharide, results in mild hindgut acidosis decreasing microbial generation of ammonia and increasing ionization to ammonium thus reducing absorption (Kircheis & Häussinger 2002). In this author’s experience oral lactulose administration at 0.3 ml/kg q 6–24 h is effective in the treatment and prophylaxis of acute and chronic HE in horses and also remains popular in human cases despite absence of a strong evidence-basis (Als-Nielsen et al 2004). The product is suitable for long-term administration (months to years) and is rarely associated with adverse effects such as soft feces.
Dietary principles in hepatic insufficiency
Basic features of the ration
Adequate digestible energy and protein consumption is very important in order to help preserve body proteins and fat stores. In human cirrhosis cases the recommended daily energy and protein intakes are respectively 147–168 kJ/kg bodyweight (BW) and 1.2 to 1.5 g/kg BW daily (Plauth et al 2006). These correspond to a small increase above field maintenance requirements for healthy horses (National Research Council [NRC] 2007a,c
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree