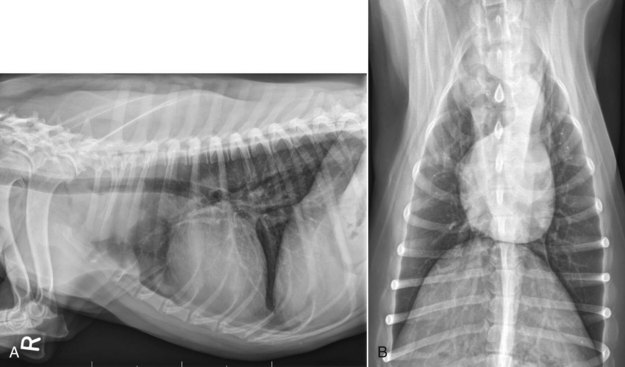
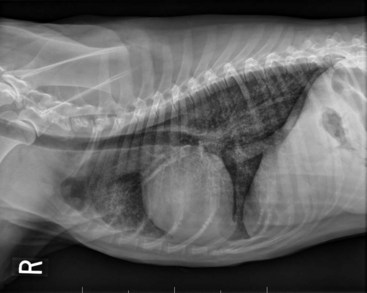
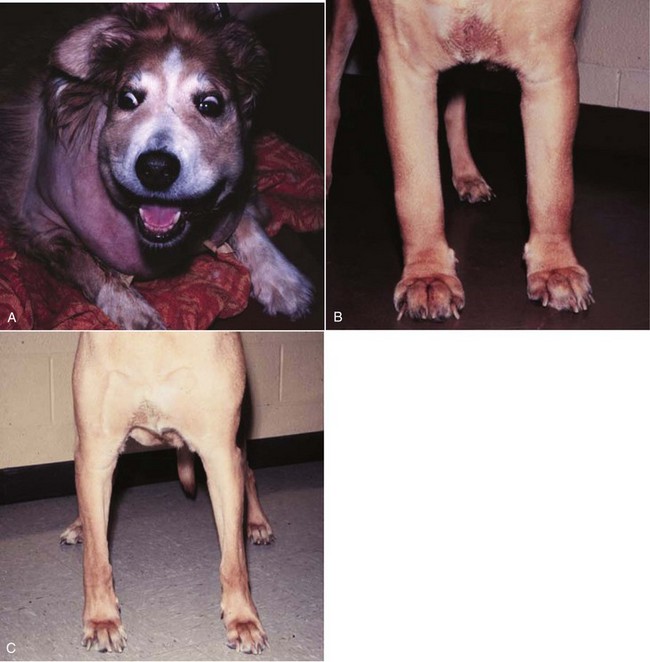
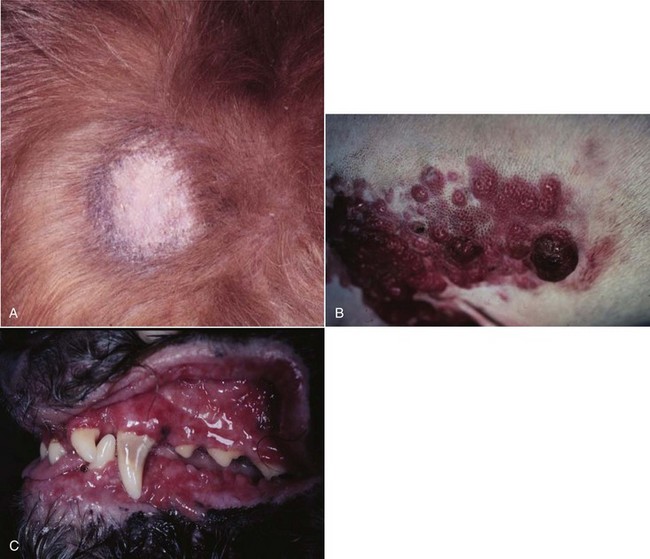
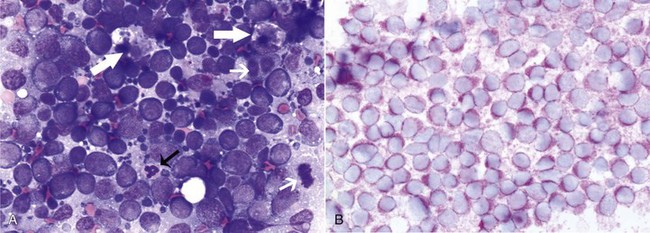
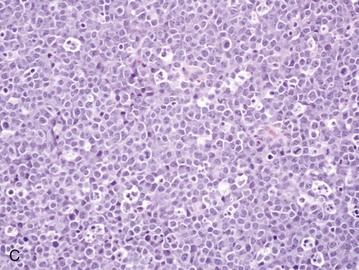
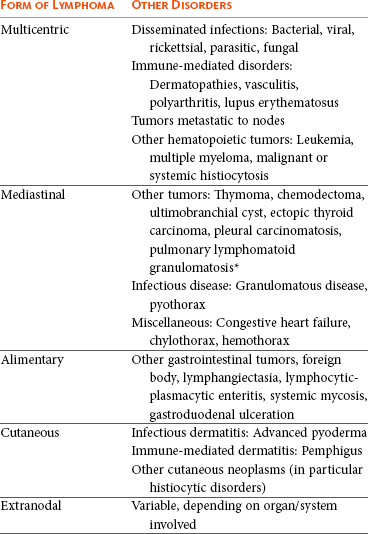
32 David M. Vail, Marie E. Pinkerton, and Karen M. Young The lymphomas (malignant lymphoma or lymphosarcoma) are a diverse group of neoplasms that have in common their origin from lymphoreticular cells. They usually arise in lymphoid tissues such as lymph nodes, spleen, and bone marrow; however, they may arise in almost any tissue in the body. Although the annual incidence of lymphoma is difficult to predict in the absence of a national canine tumor registry, it is clear that it represents one of the most common neoplasms seen in the dog. The annual incidence has been estimated to range between 13 to 24 per 100,000 dogs at risk.1–3 The annual incidence rates at specific ages are estimated to be 1.5 per 100,000 for dogs less than 1.0 year of age and 84 per 100,000 in the 10- to 11-year-old group. Lymphoma comprises approximately 7% to 24% of all canine neoplasia and 83% of all canine hematopoietic malignancies.4,5 In a review of the Veterinary Medical Data Base Program (VMDP) at Purdue University from 1987 to 1997, the frequency of canine lymphoma patients presented to 20 veterinary institutions increased from 0.75% of total case load to 2.0%, and it appears the frequency is continuing to increase. A similar trend is present in physician-based oncology; non-Hodgkin’s lymphoma (NHL) represents 5% of all new cancer cases, the fifth leading cause of cancer death, and the second fastest growing cancer in terms of mortality in humans.6 Middle-aged to older (median age of 6 to 9 years) dogs are primarily affected. A decreased risk for lymphoma is reported for intact females.7 Breeds reported to have a higher incidence include Boxers, bull mastiffs, basset hounds, St. Bernards, Scottish terriers, Airedales, and bulldogs; breeds at lower risk include dachshunds and Pomeranians.8,9 Recent advances in molecular cytogenetics (see Chapter 1, Section A), including gene microarray techniques, have been and are currently being applied to investigations of chromosomal aberrations in dogs with lymphoma.10–16 The publication of the canine genome and the commercial availability of canine gene microarrays (GeneChip Canine Genome 2.0 Array, Affymetrix, Inc.) have led to advances in our understanding of genetic events occurring in lymphoma.17 Breen’s group has documented gain of dog chromosomes 13 and 31 and loss of chromosome 14 as the most common aberrations in a group of 25 cases analyzed.11 Chromosomal aberrations have also been associated with prognosis in dogs with lymphoma. A study of 61 dogs with lymphoma demonstrated a prognostic advantage in dogs with trisomy of chromosome 13 (25% of the dogs studied) as evidenced by increase in duration of first remission and overall survival time.18 Germline and somatic genetic mutations and altered oncogene/tumor suppressor gene expression, epigenetic changes (e.g., DNA hypomethylation), signal transduction, and death-pathway alterations (e.g., Bcl-2 family) are common in human lymphomas and have been reported in the dog as well (see Chapter 1, Section A, and Chapter 14, Section B). These include N-ras, p53, Rb, and p16 cyclin-dependent kinase aberrations.19–24 Additionally, differences in the prevalence of immunophenotypic subtypes of lymphoma among different breeds indicate heritable risks.25 Additionally, telomerase activity (see Chapter 2) has been documented in canine lymphoma tissues.26–28 The hypothesis that a retrovirus may be involved in the pathogenesis of canine lymphoma has not been confirmed. However, serologic detection of Epstein-Barr virus infection, linked to some forms of lymphoma in humans, has been documented in dogs with lymphoma and is currently being investigated.29 In humans, a direct association between Helicobacter sp. infections and development of gastric lymphoma has been made.30 Although this has not been definitively shown in dogs, there is evidence of Helicobacter sp. infection in laboratory beagle dogs resulting in gastric lymphoid follicle formation that is considered a precursor of mucosa-associated lymphoid tissue (MALT) lymphoma in humans.31 In humans, evidence has accumulated implicating phenoxyacetic acid herbicides, in particular 2, 4-dichlorophenoxyacetic acid (2, 4-D), in the development of NHL. A published hospital-based case-control study of dogs indicated that owners in households with dogs that develop malignant lymphoma applied 2, 4-D herbicides to their lawn and/or employed commercial lawn care companies to treat their yard more frequently than owners of dogs without lymphoma.32 The risk of canine lymphoma was reported to rise twofold (odds ratio [OR] = 1.3) with four or more yearly owner applications of 2, 4-D. The results of this study have come under criticism, and three additional follow-up investigations have not validated assertions of increased risk.33–35 In another study, dogs exposed to lawn treatment within 7 days of application were greater than 50 times more likely to have urine levels of 2, 4-D at 50 µg/L or higher.36 The highest concentration was noted 2 days after application. In an environmental case-control study performed in Europe, two variables, residency in industrial areas and use of chemicals (defined as paints or solvents) by owners, modestly increased the risk of developing lymphoma; however, no link was found with pesticide use.37 A weak association between lymphoma in dogs and exposure to strong magnetic fields was observed in a preliminary epidemiologic study.38 In this hospital-based case-control study, the risk of developing lymphoma categorized into high or very high exposure was increased (odds ratio = 1.8). More thorough studies are necessary to evaluate this association further. Proximity to environmental waste was implicated in two European studies; however, it was felt to be a risk indicator rather than a risk factor and would require further case-control investigations.39,40 Impaired immune function has also been implicated in dogs with lymphoma. Immune system alterations in the dog such as immune-mediated thrombocytopenia, independent of age and sex, have been associated with a higher risk of subsequently developing lymphoma when compared to the normal population.41,42 Additional evidence comes from observations in human and feline transplantation patients. In a case-control study of cats undergoing renal transplant, 24% of cases developed cancer (36% of those were lymphoma) while on cyclosporine immunosuppressive therapy compared to 5.1% of control cats, none of which developed lymphoma (OR, 6.1; p = 0.001).43 A case of lymphoma developing in a dog following treatment with cyclosporine also exists.44 One report suggests an association between the immunodysregulation observed in dogs with atopic dermatitis and the risk of developing epitheliotropic T-cell lymphoma; whether this is associated with the disease or the immunomodulatory treatments commonly applied is unknown.45 Classification of malignant lymphoma in dogs is based on anatomic location, histologic criteria, and immunophenotypic characteristics. The most common anatomic forms of lymphoma, in order of decreasing prevalence, are multicentric, gastrointestinal (GI), mediastinal, and cutaneous forms.46 Primary extranodal forms, which can occur in any location outside the lymphatic system, include the eyes, central nervous system (CNS), bone marrow, bladder, heart, and nasal cavity. The pathologic characteristics of the various anatomic classifications will be discussed in this section and clinical characteristics will be described in subsequent sections. Eighty-four percent of dogs with lymphoma develop the multicentric form, which is usually characterized by the presence of superficial lymphadenopathy (Figure 32-1).46 The alimentary form of lymphoma is much less common, accounting for 5% to 7% of all canine lymphomas. This form is reported to be more common in male dogs than female dogs.6 Primary GI lymphoma in dogs may occur focally but more often affects multiple segments, with thickening of the wall, narrowing of the lumen, and frequently mucosal ulceration.47,48 Histologically, there is infiltration of neoplastic lymphocytes throughout the mucosa and submucosa, with occasional transmural infiltration. Liver and local lymph nodes are often secondarily involved. Lymphocytic-plasmacytic enteritis (LPE) can be seen adjacent to or distant from the primary tumor. Pathologically, some of these neoplasms may resemble plasma cell tumors, and aberrant production of immunoglobulins may occur. Histopathologically, distinguishing between GI lymphoma and LPE can be difficult. Some have suggested that LPE may be a prelymphomatous change in the GI tract. A syndrome of immunoproliferative intestinal disease characterized by LPE has been described in Basenjis, which subsequently develop GI lymphoma.49 In addition, plasma cell–rich areas with heterogeneous lymphomatous infiltration may resemble lesions of LPE. Only a few reports specifically identify the immunophenotype of the lymphocyte subpopulations in alimentary lymphoma in dogs. Historically, it was presumed that they most likely originate from B cells; however, recent evidence suggests that most GI lymphomas in dogs arise from T cells and often exhibit epitheliotropism.48,50 The Boxer and Shar-pei breeds may be overrepresented in cases of alimentary lymphoma.50,51 The mediastinal form of the disease occurs in approximately 5% of cases.46 This form is characterized by enlargement of the cranial mediastinal lymph nodes, thymus, or both (Figure 32-2). Hypercalcemia is reported to occur in 10% to 40% of dogs with lymphoma and is most common with the mediastinal form. In a study of 37 dogs with lymphoma and hypercalcemia, 16 (43%) had mediastinal lymphoma.52 The mediastinal form in dogs is most commonly associated with a T-cell phenotype.53,54 Cutaneous lymphoma can be solitary or more generalized and usually is classified as epitheliotropic (mycosis fungoides) or nonepitheliotropic.55 Canine epitheliotropic cutaneous lymphoma originates from T-cells,55–60 similar to its development in humans. In dogs, these more commonly represent CD8+ cells, whereas in humans they are typically CD4+ cells. A rare form of cutaneous T-cell lymphoma, characterized by skin involvement with evidence of peripherally circulating large (15 to 20 µm in diameter) malignant T-cells with folded, grooved nuclei, has been described. In humans, this is referred to as Sézary syndrome and has been reported in both dogs and cats.61–63 Nonepitheliotropic cutaneous lymphomas form single or multiple dermal or subcutaneous nodules or plaques; histologically, they spare the epidermis and papillary dermis and affect the middle and deep portions of the dermis and subcutis.55 Hepatosplenic lymphoma is a relatively uncommon, distinct presentation in the dog marked by a lack of significant peripheral lymphadenopathy in the face of hepatic, splenic, and bone marrow infiltration with malignant lymphocytes, usually of T-cell origin.64,65 Biologically, this form of lymphoma is extremely aggressive and poorly responsive to therapy. In humans the tumor usually is composed of γδT-cells (i.e., T-cells that express the γδT-cell receptor), and this immunophenotype has been confirmed in at least one dog in the veterinary literature.65 Intravascular (angiotropic, angioendotheliomatosis) lymphoma is a distinct form of lymphoma defined as proliferations of neoplastic lymphocytes within the lumen and wall of blood vessels in the absence of a primary extravascular mass or leukemia. It has been reported several times in the veterinary literature, and in most cases it involves the CNS and peripheral nervous system (PNS), including the eye.66–71 The B-cell immunophenotype is most common in humans; however, in most reported cases in dogs, the origin is either T-cell or null cell (neither B- nor T-cell), although one case of a B-cell phenotype has been reported. Pulmonary lymphomatoid granulomatosis (PLG) is a rare pulmonary infiltrative and/or nodular disorder characterized by a heterogenous accumulation of lymphocytes (both B and T, although some evidence suggests primarily a T-cell origin), neutrophils, plasma cells, and macrophages, often arranged angiocentrically.72–75 Whether this syndrome is a true lymphoma or a prelymphoma state is debatable. Clinical signs are related to respiratory compromise, and various chemotherapeutic protocols have been used with reported results varying from rapid progression to long-term clinical remissions. Lymphomas arise from clonal expansion of lymphoid cells with distinctive morphologic and immunophenotypic features. Many histologic systems have been used to classify NHL in humans, and some of these have been applied to lymphoma in the dog and other species. The National Cancer Institute (NCI) Working Formulation76 and the updated Kiel system77 have been adapted to canine tumors with some success. The World Health Organization (WHO) also publishes a histologic classification scheme, which uses the revised European American lymphoma (REAL) system as a basis for defining histologic categories of hematopoietic and lymphoid tumors in domestic animals.78 This system incorporates anatomic, histologic, and immunophenotypic criteria (B- and T-cell immunophenotype), with the goal of enabling accurate and reproducible diagnosis of specific neoplastic disease entities. This theoretically should assist in better tailoring of treatment protocols, better correlation of prognosis, and better comparative capabilities. Table 32-1 shows some of the WHO categories in three different surveys, including a 2-year survey (2008-2009) of canine necropsy and biopsy cases at the University of Wisconsin-Madison Veterinary Medical Teaching Hospital pathology service79,80; some of the less common categories in the WHO system were not represented and are not listed. The WHO system provides accurate and consistent reproducible diagnostic results similar to the system used in human pathology; accuracy among a group of pathologists examining 300 cases was at 83% agreement, and accuracy in evaluating the six most common diagnoses (80% of the cases) was 87%.81 Clinical studies are needed to correlate the various categories of disease with biologic behavior, response to treatment, and prognosis. Preliminary results indicate dogs with indolent lymphoma (e.g., marginal zone lymphoma, follicular lymphoma, B- or T-cell small cell lymphoma, T-cell–rich B-cell lymphoma, and T zone lymphoma) maintain normal activity and appetite levels even during advanced stages of disease and experience long-term survival even with limited or no therapy.81–84 Table 32-1 World Health Organization Classification System for Canine Lymphoma †Three B-cell and four T-cell cutaneous lymphomas, not specified as epitheliotropic/nonepitheliotropic. ‡Includes one T-zone lymphoma. Modified from Sueiro FAR, Alessi AC, Vassallo J: Canine lymphomas: A morphological and immunohistochemical study of 55 cases, with observations on p53 immunoexpression, J Comp Pathol 131:207–213, 2004; and Vezzali E, Parodi AL, Marcato PS, et al: Histopathological classification of 171 cases of canine and feline non-Hodgkin lymphoma according to the WHO, Vet Comp Oncol 8:36–49, 2009. The Working Formulation (WF) was developed to allow investigators to “translate” among the numerous classification systems so that clinical trials could be compared in humans. Most of the larger compilations agree that most canine lymphomas are intermediate or high grade; however, diffuse immunoblastic forms appear to predominate in the United States, whereas the follicular large cell variations predominate in Europe. A comparison of European and American classifications is warranted based on this discrepancy. The WF categorizes tumors according to pattern (diffuse or follicular) and cell type (e.g., small cleaved cell, large cell, immunoblastic), but it does not include information about the immunophenotype of the tumor.76 The WF subtypes are related to the biology of the tumor and patient survival. The updated Kiel classification includes the architectural pattern, morphology (centroblastic, centrocytic, or immunoblastic), and immunophenotype (B-cell or T-cell) of the tumor cells.77 In both systems, the tumors can then be categorized as low-grade, intermediate grade, or high-grade malignancies. Low-grade lymphomas composed of small cells with a low mitotic rate typically progress slowly and are associated with long survival times but are ultimately incurable. High-grade lymphomas with a high mitotic rate progress rapidly but are more likely to respond initially to chemotherapy and, in humans, are potentially curable. Several features of canine lymphomas become apparent when these classification systems are applied. The most striking difference between canine and human lymphomas is the scarcity of follicular lymphomas in the dog.79,80 Some diffuse lymphomas in the dog may initially be follicular, but these may progress to the more aggressive, diffuse form by the time of diagnostic biopsy. The most common form of canine lymphoma is diffuse large-cell lymphoma, a high-grade tumor most commonly of B-cell origin.80,81,85 Only a small percentage of canine lymphomas (5.3% to 29%) are considered low-grade tumors. High-grade lymphomas occur frequently if diffuse large-cell lymphomas, classified as intermediate grade in the WF, are considered high-grade, as in the updated Kiel classification (in which they are labeled as diffuse centroblastic lymphomas). A documented difference exists in the prevalence of the various immunophenotypes based on breed.25 For example, cocker spaniels and Doberman pinschers are more likely to develop B-cell lymphoma, Boxers are more likely to have T-cell lymphoma, and golden retrievers appear to have an equal likelihood of B- and T-cell tumors. To be clinically useful, these classification systems in the end must yield information about response to therapy, maintenance of remission, and survival. Some studies suggest that the subtypes in the WF can be correlated with survival, and the Kiel system may be useful for predicting relapse.86,87 In most studies, high-grade lymphomas achieve a complete response (CR) to chemotherapy significantly more often than low-grade tumors. However, dogs with low-grade tumors may live a long time without aggressive chemotherapy.83,84 Dogs with T-cell lymphomas have shown a lower rate of CR to chemotherapy and shorter remission and survival times than dogs with B-cell tumors (with the exception of low-grade T-cell subtypes).53,54,86,88 Furthermore, T-cell lymphomas tend to be associated with hypercalcemia.89,90 In the veterinary literature, 60% to 80% of canine lymphomas are of B-cell origin; T-cell lymphomas account for 10% to 38%; mixed B- and T-cell lymphomas account for as many as 22%; and null cell tumors (i.e., neither B-cell nor T-cell immunoreactive) represent fewer than 5%.53,54,91–93 The development of monoclonal antibodies to detect specific markers on canine lymphocytes has made immunophenotyping of tumors in dogs routinely available in many commercial laboratories. Such techniques can be performed on paraffin-embedded samples, from tissue microarrays, on cytologic specimens obtained by fine-needle aspiration (FNA) of lesions, or by flow cytometric analysis of cellular fluid samples (e.g., peripheral blood, effusions) and lesion aspirates. The Rappaport classification system, proposed in 1956 for human NHL, describes the architectural pattern (follicular or diffuse) and the cytologic features (well differentiated, poorly differentiated, or histiocytic) of lymphoma.94,95 This system has not proved useful in providing prognostic information or in guiding therapy in dogs with lymphoma because of the low number of follicular lymphomas in dogs, the problematic “histiocytic” subgroup, and the failure to account for different morphologic and immunologic cell types. One criticism of the Rappaport, Kiel, and WF classification systems is that they fail to include extranodal lymphomas as a separate category. The WHO system does include anatomic location as a factor in determining certain categories. Although differences between nodal and extranodal tumors in biologic behavior and prognosis are well recognized, comparative information about the histogenesis of these tumors is lacking. For example, in humans small-cell lymphomas arising from MALT are composed of cells with a different immunophenotype than that of other small-cell lymphomas (i.e., MALT lymphomas typically are negative for both CD5 and CD10). Except for cutaneous lymphoid neoplasms, detailed characterization of extranodal lymphomas in dogs has not been done. Although cutaneous lymphoma is a heterogeneous group of neoplasms that includes an epitheliotropic form resembling mycosis fungoides and a nonepitheliotropic form, most cutaneous lymphomas have a T-cell phenotype.64,96 The clinical signs associated with canine lymphoma are variable and depend on the extent and location of the tumor. Multicentric lymphoma, the most common form, is usually distinguished by the presence of generalized painless lymphadenopathy (see Figure 32-1). Enlarged lymph nodes are usually painless, rubbery, and discrete and may initially include the mandibular and prescapular nodes. In addition, hepatosplenomegaly and bone marrow involvement occur commonly. Most dogs with multicentric lymphoma present without dramatic signs of systemic illness (WHO substage a) (Box 32-1); however, a large array of nonspecific signs such as anorexia, weight loss, vomiting, diarrhea, emaciation, ascites, dyspnea, polydipsia, polyuria, and fever can occur (WHO substage b). Dogs presented with T-cell lymphoma are more likely to have constitutional (i.e., substage b) signs. Polydipsia and polyuria are particularly evident in dogs with hypercalcemia of malignancy. Dogs may also be presented with clinical signs related to blood dyscrasias secondary to marked tumor infiltration of bone marrow (myelophthisis) or paraneoplastic anemia, thrombocytopenia, or neutropenia. These could include fever, sepsis, anemia, and hemorrhage. Diffuse pulmonary infiltration is seen in 27% to 34% of dogs with the multicentric form, as detected by radiographic changes (Figure 32-3).97,98 Based on bronchoalveolar lavage, the actual incidence of lung involvement may be higher.99,100 Dogs with GI or alimentary lymphoma are usually presented with nonspecific GI signs, such as vomiting, diarrhea, weight loss, and malabsorption.47,101,102 Mesenteric lymph nodes, spleen, and liver may be involved. The mediastinal form of lymphoma is characterized by enlargement of the cranial mediastinal structures and/or thymus (see Figure 32-2), and clinical signs are associated with the extent of disease with resulting respiratory compromise or polydipsia/polyuria from hypercalcemia. Commonly, dogs are presented with respiratory distress caused by a space-occupying mass and pleural effusion, exercise intolerance, and possibly regurgitation. Additionally, dogs with mediastinal lymphoma may present with precaval syndrome, characterized by pitting edema of the head, neck, and forelimbs secondary to tumor compression or invasion of the cranial vena cava (Figure 32-4). Signs in dogs with extranodal lymphoma depend on the specific organ involved. Cutaneous lymphoma is usually generalized or multifocal.55–57 Tumors occur as nodules, plaques, ulcers, and erythemic or exfoliative dermatitis with focal hypopigmentation and alopecia. Epitheliotropic T-cell lymphoma (e.g., mycosis fungoides) typically has a clinical course with three apparent clinical stages. Initially, there will be scaling, alopecia, and pruritus (Figure 32-5, A), which can mimic a variety of other skin conditions. As the disease progresses, the skin becomes more erythematous, thickened, ulcerated, and exudative. The final stage is characterized by proliferative plaques and nodules with progressive ulceration (Figure 32-5, B). Oral involvement may also occur and this can appear as multicentric erythematous plaque-like lesions or nodules associated with the gum and lips (Figure 32-5, C). Extracutaneous involvement can also occur, most often in the lymph nodes, spleen, liver, and bone marrow. Nonepitheliotropic cutaneous lymphomas form single or multiple dermal or subcutaneous nodules or plaques; histologically, they spare the epidermis and papillary dermis and affect the middle and deep portions of the dermis and subcutis.55 Dogs with primary CNS lymphoma may be presented with either multifocal or solitary involvement.103–105 Seizures, paralysis, and paresis may be noted. Ocular lymphoma is characterized by infiltration and thickening of the iris, uveitis, hypopyon, hyphema, posterior synechia, and glaucoma.106,107 In one study of 94 cases of canine multicentric lymphoma, 37% had ocular changes consistent with lymphoma, and in a series of 102 cases of uveitis in dogs, 17% were secondary to lymphoma.107 Anterior uveitis was most commonly seen in advanced stage of disease (stage V). Dogs with intravascular lymphoma usually present with signs relative to CNS, PNS, or ocular involvement.66–71 These include paraparesis, ataxia, hyperesthesia, seizures, blindness, lethargy, anorexia, weight loss, diarrhea, polyuria, polydipsia, and intermittent fever. Finally, dogs with pure hepatosplenic lymphoma usually are presented with nonspecific signs of lethargy, inappetence, and weakness and often are icteric.64,65 The differential diagnosis of lymphadenopathy depends on the dog’s travel history (i.e., relative to infectious disease) and the size, consistency, and location of affected lymph nodes. Other causes of lymphadenopathy include infections caused by bacteria, viruses, parasites (Toxoplasma sp., Leishmania sp.), rickettsial organisms (Salmon-poisoning, Ehrlichia sp.), and fungal agents (Blastomyces and Histoplasma sp.). The potential for hypercalcemia to accompany systemic fungal diseases may further complicate differentiation from lymphoma. Discrete, hard, asymmetric lymph nodes, particularly if they are fixed to underlying tissues, may indicate metastatic tumors such as mast cell tumor or carcinoma. Immune-mediated diseases (e.g., pemphigus, systemic lupus erythematosus) also may result in mild-to-moderately enlarged lymph nodes. The various differential diseases or conditions that can resemble canine lymphoma are listed in Table 32-2. Table 32-2 Differential Diseases or Conditions That Can Resemble Canine Lymphoma *The existence of this disease is controversial; in most cases, the disease has been reclassified as a lymphoid neoplasm. Canine lymphoma also may be associated with paraneoplastic syndromes (see Chapter 5). Anemia is the most common lymphoma-related paraneoplastic syndrome.108 Paraneoplastic hypercalcemia is also common and is characterized clinically by anorexia, weight loss, muscle weakness, lethargy, polyuria, polydipsia, and rarely CNS depression and coma. Lymphoma-induced hypercalcemia in most cases results from parathyroid hormone–related peptide (PTHrP), elaborated by neoplastic cells; however, it can also be related to the production of several other humoral factors, including interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β), and vitamin D analogs (e.g., 1,25-dihydroxyvitamin D).89,109,110 As previously discussed, hypercalcemia is most commonly associated with the T-cell immunophenotype. Other paraneoplastic syndromes that may be encountered include monoclonal gammopathies, neuropathies, and cancer cachexia. A thorough physical examination should include palpation of all assessable lymph nodes, including a rectal examination; in the authors’ experience, a significant proportion of dogs will have rectal polyps consisting of aggregates of neoplastic lymphocytes. Inspection of mucous membranes for pallor, icterus, petechiae, and ulceration should be undertaken as these signs may indicate anemia or thrombocytopenia secondary to myelophthisis or immune-mediated disease or may be evidence of major organ failure or uremia. Abdominal palpation may reveal organomegaly, intestinal wall thickening, or mesenteric lymphadenopathy. The presence of a mediastinal mass and/or pleural effusion can be suspected following thoracic auscultation. An ocular examination, including funduscopic assessment, may reveal abnormalities (e.g., uveitis, retinal hemorrhage, ocular infiltration) in approximately one-third to one-half of dogs with lymphoma.107,111 Anemia, the most common lymphoma-related hematologic abnormality, is usually normochromic and normocytic (nonregenerative), consistent with anemia of chronic disease.108 However, hemorrhagic and hemolytic anemias may also occur, and regenerative anemias may reflect concomitant blood loss or hemolysis. Additionally, if significant myelophthisis is present, anemia may be accompanied by thrombocytopenia and leukopenia.112,113 In animals with anemia or evidence of bleeding, in addition to a platelet count, a reticulocyte count and coagulation testing may be indicated. Thrombocytopenia may be seen in 30% to 50% of cases, but bleeding is seldom a clinical problem. Neutrophilia can be seen in 25% to 40% of dogs and lymphocytosis occurs in approximately 20% of affected dogs.108 Circulating atypical lymphocytes may be indicative of bone marrow involvement and leukemia. It is important to differentiate multicentric lymphoma with bone marrow involvement (i.e., stage V disease) from primary lymphoblastic leukemia (see later), as the prognosis for each is entirely different. Hypoproteinemia is observed more frequently in animals with alimentary lymphoma. In dogs with a high total protein or evidence of an increased globulin fraction on a chemistry profile, serum proteins may be evaluated by serum electrophoresis. Monoclonal gammopathies have been reported to occur in approximately 6% of dogs with lymphoma.114 Serum biochemical abnormalities often reflect the anatomic site involved, as well as paraneoplastic syndromes such as hypercalcemia. In cases of hypercalcemia of unknown origin, lymphoma should always be considered high on the differential disease list, and diagnostic testing directed at this possibility should be undertaken (see Chapter 5). In addition, the presence of hypercalcemia can serve as a biomarker for response to therapy and early recurrence. Increased urea nitrogen and creatinine concentrations can occur secondary to renal infiltration with tumor, hypercalcemic nephrosis, or prerenal azotemia from dehydration. Increases in liver-specific enzyme activities or bilirubin concentrations may result from hepatic parenchymal infiltration. Increased serum globulin concentrations, usually monoclonal, occur infrequently with B-cell lymphoma. Several abnormalities in serum have been explored as biomarkers of lymphoma in the dog. Examples include alpha-fetoprotein, alpha-1 glycoprotein levels, zinc, chromium, iron, endostatin, vascular endothelial growth factor (VEGF), lactate dehydrogenase, C-reactive protein haptoglobin, and antioxidants/oxidative stress markers.115–122 The clinical, biologic, and prognostic significance of these alterations is yet to be definitively characterized. Morphologic examination of the tissue and cells that constitute the tumor is essential to the diagnosis of lymphoma. Care should be taken to avoid lymph nodes from reactive areas (e.g., mandibular lymph nodes), unless those nodes are the only ones enlarged; the prescapular or popliteal lymph nodes are preferable if also involved. Also, lymphoid cells are fragile, and in preparing smears of aspirated material only gentle pressure should be applied in spreading material on the slides. In most cases, a diagnosis of lymphoma can be made on evaluation of fine-needle aspirates of affected lymph nodes or other tissues. Typically, most of the cells are large lymphoid cells (>2 times the diameter of a red blood cell [RBC] or larger than neutrophils), and they may have visible nucleoli and basophilic cytoplasm (Figure 32-6, A) or fine chromatin with indistinct nucleoli. Because tissue architecture is not maintained in cytologic specimens, effacement of the node or capsular disruption cannot be detected. Therefore marked reactive hyperplasia characterized by increased numbers of large lymphoid cells may be difficult to distinguish from lymphoma, and small cell lymphomas may have few cytologic clues that point to malignancy. Also, classification of lymphoma, which has been attempted using cytologic appearance and immunophenotypic analysis,123 into subcategories that make up the low-, intermediate-, and high-grade forms is performed most accurately on histologic sections (discussed previously).
Hematopoietic Tumors
 Section A
Section A
Canine Lymphoma and Lymphoid Leukemias
Lymphoma
Etiology
Genetic and Molecular Factors
Infectious Factors
Environmental Factors
Immunologic Factors
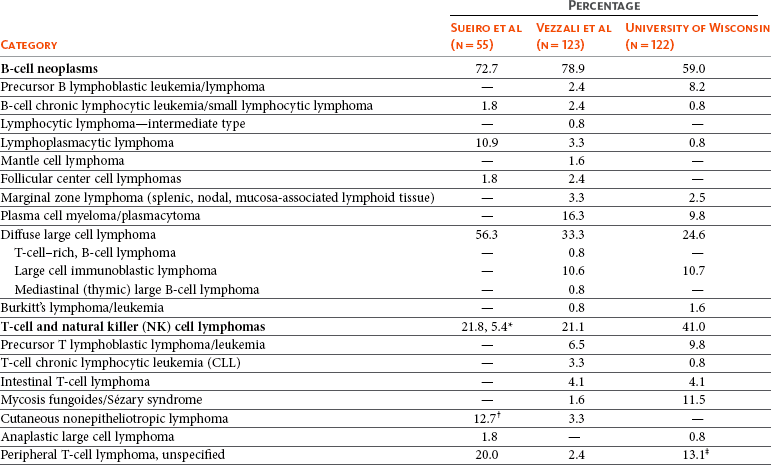
Classification and Pathology
Atypical Anatomic Forms of Lymphoma
Histologic Classification Systems
History and Clinical Signs
Diagnostics and Clinical Staging
Physical Examination
Complete Blood Count, Biochemistry Profile, and Urinalysis
Histologic and Cytologic Evaluation of Lymph Nodes
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree