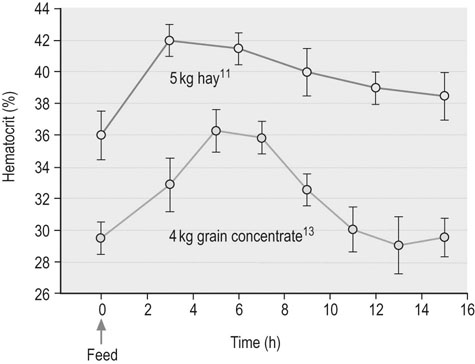
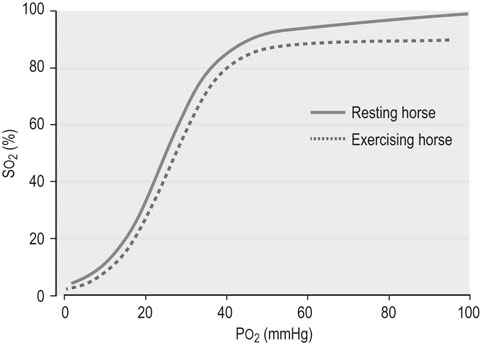
The circulation of blood and its many components to and from the major organs, including the exercising musculature, represents one of the most critical aspects of exercise physiology. Since evaluation of the hemogram and serum or plasma biochemistry analyzes can provide insights into oxygen transport, organ function, and fluid, electrolyte and acid–base balance, it is not surprising that such tests currently rank among the most frequent diagnostic techniques employed during investigation of suboptimal performance or disease in equine athletes.1,2 Although these methods provide a broad assessment of possible inflammation or dysfunction of various organ systems, training and exercise both can influence multiple hematologic and biochemistry variables in healthy horses. Therefore a comprehensive understanding of expected changes in response to these stimuli is essential for accurate interpretation of laboratory analyzes. In addition, among other factors, sample collection and processing can substantially impact analysis results; thus, exemplary sample collection, handling and processing techniques should always be employed.3 Blood for hematologic analysis should be collected from the jugular or cephalic vein atraumatically to minimize hemolysis or activation of platelets and clotting factors.3 Blood should be obtained into an EDTA tube, which is subsequently inverted gently multiple times, with inspection for clumping or clotting. In horses in which an accurate platelet count is desirable, an additional sample should be collected using sodium citrate as the anticoagulant rather than EDTA, to prevent platelet clumping and subsequent pseudothrombocytopenia. Since even relatively brief delays in processing can result in deterioration of equine leukocyte morphology, samples to be transported for analysis should be accompanied by a thin blood smear prepared at the time of collection.3 The use of automated cytometers in clinical practice has expanded rapidly in the last few years and has greatly improved the convenience of performing on-site hematologic analysis.3 However, microscopic examination of a stained blood film should always accompany automated analysis to verify the accuracy of reported leukocyte, red cell and platelet counts, and to ensure an accurate differential cell count and description of cellular morphology is obtained.3 This is particularly critical for diseased patients in which abnormal cell counts and distributions are considered likely or in which abnormalities of cell morphology might be present.3 In addition a spun hematocrit should be performed, and plasma or serum total protein values obtained by refractometry should be interpreted with caution in patients in which hyperlipemia is possible since falsely high values may be obtained.3 The spun hematocrit represents one of the most accurate and reproducible methods of determining blood erythrocyte concentrations in horses, providing the EDTA tube into which the blood sample is obtained is appropriately filled to avoid osmotic shrinkage of the cells, the blood sample is appropriately mixed before filling the microhematocrit tubes, and the microhematocrit tube is appropriately centrifuged and correctly interpreted.3 Quantitative biochemical analyzes can be performed on serum or plasma, depending on the preference of the analyzing laboratory or the specific equipment to be used for in-clinic analyzes.3 Plasma may be preferable in emergency situations to facilitate more timely results. Heparinized plasma should be used for biochemical analyzes rather than EDTA-preserved plasma to avoid erroneous results, particularly for concentration of specific electrolytes such as calcium (which might be below artifactually low) and potassium (which might be artifactually high). Whole blood samples should be centrifuged within 30 minutes followed by separation of serum or plasma to reduce changes associated with ongoing red cell metabolism. Samples specifically designated for plasma lactate analysis can be collected into sodium fluoride tubes and chilled if lactate concentrations will not be obtained from gas analysis of heparinized whole blood.3 Blood intended for chemistry analyzes, which will include measurement of serum glucose concentrations, can be preserved with appropriate quality by collection into serum separator tubes or for samples intended only for measurement of glucose concentration, tubes containing sodium fluoride or a similar inhibitor of glycolysis.4 However, changes in multiple blood chemistry variables should be expected even in separated samples if they are stored at room temperature for 24 hours or more.5 Therefore, if prolonged delays in sample analysis are anticipated, refrigeration of samples at 4°C is appropriate prior to biochemical or hematological analyzes, after preparation of an accompanying slide to preserve original cell morphology.3,4 Samples for venous or arterial blood gas analysis should be collected into evacuated glass tubes, or into a 1 or 3 mL glass or quality plastic syringe containing minimal retained heparin volume, and which is capped immediately after the sample is obtained.3 The use of plastic heparinized vacutainer tubes results in substantial increases in the oxygen content of equine blood samples, accompanied by lesser alterations in carbon dioxide content, and should therefore be avoided.6 Arterial blood is the ideal sample to determine blood oxygenation status and cardiorespiratory function, though venous blood gas analysis can provide convenient information regarding metabolic disorders and electrolyte status. The transverse facial artery provides the most convenient site for arterial blood sampling in the standing adult horse. Analysis of both venous and arterial samples should be performed as rapidly as possible after collection. A variety of factors must be taken into account when evaluating the results of common laboratory analyzes, particularly so for the complete blood count.7 These factors include the horse’s age and breed, the stage of the daily routine in relationship to the timing of blood collection, and the horse’s demeanor at the time of blood collection.7 Differences generated by these various factors are described in more detail below, but are usually mild to moderate in degree, and unlikely to result in values outside of appropriate reference limits. However, these factors are particularly relevant when repeated analyzes are performed within the same individual, and therefore attention should be paid to the timing of blood sampling in relationship to the daily routine when serial sampling is expected.8 Conditioning of horses stimulates a variety of changes, including endocrine alterations directed at increasing plasma volume and defending tonicity, which may result in changes in hematologic and biochemical variables over time in training individuals, or in differences in results obtained from fit versus unfit individuals.9 Plasma volume expansion likely peaks within eight weeks of submaximal training in the horse, and is likely more pronounced in younger horses.10 Reference intervals for a variety of equine breeds and activity groups are outlined in Appendices 1 and 2. Consumption of a relatively large meal (4–5 kg) of forage or concentrate can also elicit substantial changes in plasma volume associated with increased saliva production and extracellular fluid shifts associated with digestion (Fig. 42.1).11,12 Packed cell volume (PCV) has been reported to increase as much as 16% from pre-feeding values, peaking one to five hours after feeding.11,13 Comparable but more transient increases in plasma total protein concentration may occur, in addition to transient mild increases in serum sodium and decreases in serum potassium concentrations. These phenomena reflect a decline in plasma volume with feed ingestion and the effects of subsequent aldosterone release.12,13 Changes are reported to be more pronounced in horses receiving intermittent large meals compared to horses eating more frequent meals.11,12 However, a study of feed ingestion and voluntary sodium intake in conditioned athletic horses did not identify significant differences in PCV or plasma aldosterone concentrations when horses were fed two times compared to six times per day, though minor transient increases in plasma protein concentration and plasma osmolality were observed after horses consumed a large meal.14 It was concluded in this study that voluntary sodium intake had the most significant impact on fluid and electrolyte regulation in conditioned horses, and that feeding frequency had minimal influence. It is possible that the differences between the findings of these studies reflect the superior capacity of conditioned individuals to preserve plasma volume in the face of challenges such as exercise and feeding.14 However, even in conditioned individuals participating in a twice-daily feeding schedule, it remains prudent to obtain samples for hemogram analysis prior to a meal or several hours after a meal to reduce the possible effects of feed ingestion on subsequent results. Excitability or apprehension at the time of blood sampling can result in catecholamine-mediated splenic contraction, producing substantial increases in PCV.15 Increases in leukocyte and platelet numbers can also occur since these blood components are also sequestered in the spleen. Obtaining a blood sample quickly and calmly within 30 seconds of encountering the horse will help circumvent catecholamine-related effects. Therefore if blood collection is anticipated as part of the assessment of a performance horse, it best precedes more extensive physical examination procedures in nervous horses in particular.16 In addition, recent exercise can increase PCV and peripheral leukocyte counts, and can alter the neutrophil : lymphocyte ratio.8,17–20 Hence, where possible, blood samples are likely best collected in the morning prior to exercise. Similarly, transport and tranquilization can also affect complete blood count results and therefore sample collection should be temporally distant from such events. Finally, the clinician should ensure that results are interpreted in light of individual reference limits specific to the laboratory or equipment involved in the analyzes, and that reference limits are in comparable units for all provided results. Where possible, reference limits relevant to the age, breed and conditioning of the horse being examined should also be taken into account (Appendices 1 and 2). Erythrocytes are anucleate cells which primarily function to transport oxygen to the tissues, carbon dioxide to the lungs, and to buffer hydrogen ions in the blood, all largely as a result of their substantial hemoglobin content. On average, there are approximately 9 × 106 mature erythrocytes per microliter of equine blood, with a circulating life span of approximately 150 days.3 In comparison to some of the other domestic species, horses do not usually have circulating reticulocytes (immature erythrocytes with stainable cytoplasmic RNA) even during regenerative anemia states; however, some hematology analyzers will report low concentrations of reticulocytes in equine blood.3 A common measure of erythrocyte mass applied in the horse is the hematocrit (frequently used synonymously with PCV), which represents the percentage of the blood volume filled with erythrocytes.3 This value can be calculated from the mean cell volume (MCV) and erythrocyte concentration, in which case it is often referred to as hematocrit or calculated hematocrit, or else it can be determined from measurement of a centrifuged microhematocrit tube, in which case it is often referred to as the packed cell volume (PCV) or spun hematocrit. Hematocrit values usually reliably reflect blood hemoglobin and erythrocyte concentrations in the horse, though it should be recognized that blood hemoglobin concentration represents the most direct measure of the blood’s capacity to carry oxygen.3 Though plasma contains a small amount of dissolved oxygen (3 mL per liter of blood) this is inadequate to meet normal tissue oxygen demands, let alone the dramatically enhanced oxygen demands associated with exercise. Hence oxygen transport in the blood is achieved primarily via the reversible combination of hemoglobin and oxygen molecules within erythrocytes, which increases the oxygen-carrying capacity of the blood to above 200 mL per liter.21 The high pressure of oxygen (~100 mmHg) within the alveolar air of the lung facilitates rapid diffusion of oxygen into the less well oxygenated pulmonary capillary blood (PO2 ~40 mmHg) and subsequently into the erythrocytes, where it saturates the four oxygen-binding sites of each hemoglobin molecule. The relationship between the partial pressure of oxygen in the blood and the oxygen saturation of hemoglobin is represented by the oxygen–hemoglobin dissociation curve (Fig. 42.2).22 The affinity of hemoglobin for oxygen progressively increases as successive oxygen molecules bind, reflected by the steep aspect of the sigmoidal curve. However, at oxygen pressures exceeding 50 to 60 mmHg, the curve flattens, representing a plateau in oxygen saturation that occurs in spite of an increasing partial pressure of oxygen in the blood.21 The characteristics of this system promote the release of oxygen molecules to the tissues when PO2 is low, such as in the peripheral tissue capillaries, while oxygen saturation of the blood can be maintained at the pulmonary capillary level even if alveolar oxygen pressure declines to some degree. Directional shifts in the oxygen–hemoglobin dissociation curve can occur and represent changes in the affinity of hemoglobin for oxygen. A rightward shift in the curve reflects a decreased affinity of hemoglobin for oxygen, and is elicited by increases in body temperature, plasma hydrogen ion concentration, plasma CO2 partial pressure, and the concentration of 2,3-bisphosphoglycerate in the erythrocytes.3,21 These changes enhance the dissociation of oxygen from hemoglobin and therefore facilitate the subsequent distribution of oxygen to the tissues. Many of these changes are provoked by exercise, consequently causing a rightward shift in the oxygen–hemoglobin dissociation curve and enhancing the delivery of oxygen to contracting muscle tissue. Carbon dioxide is transported in the blood as dissolved CO2 (~5%), bicarbonate ions (~70–90%), and carbamate bound to hemoglobin and plasma proteins (~5–10%).21 However, it should be recognized that the proportions of these various states is very dynamic, and that the contributions of each likely change substantially during exercise activity.23 Carbon dioxide transport is coupled closely with oxygen transport to the benefit of both systems, as demonstrated in Figure 42.3. Carbon dioxide produced by the tissues diffuses into the tissue capillaries and subsequently the erythrocytes, facilitating the release of oxygen molecules from hemoglobin to the tissues. Within the erythrocytes, CO2 is converted to bicarbonate ions and protons in a process greatly facilitated by the high carbonic anhydrase activity within red cells, and accommodated by a shift of chloride ions into the red cells in exchange for bicarbonate. The greater affinity of reduced hemoglobin for protons compared to oxyhemoglobin facilitates this process and enables transport of a large proportion of the carbon dioxide load from the tissues to the lungs in the form of plasma bicarbonate, though the contribution of dissolved CO2 to gas exchange also increases substantially during exercise.23
Hematology and serum biochemistry of the equine athlete
Introduction
Methods
Hematology: structure and function
Erythrocytes and gas exchange
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine
