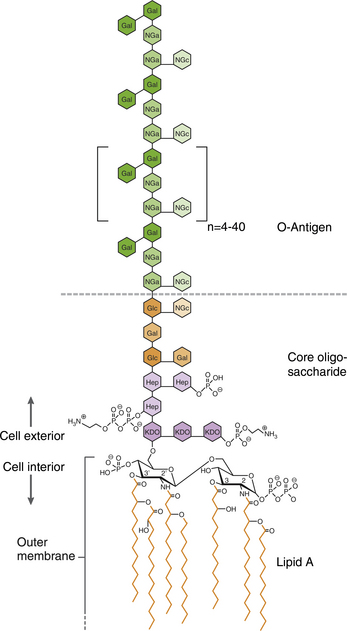
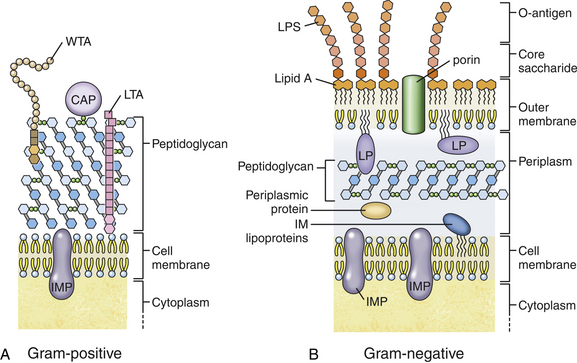
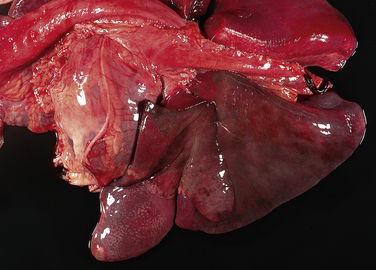
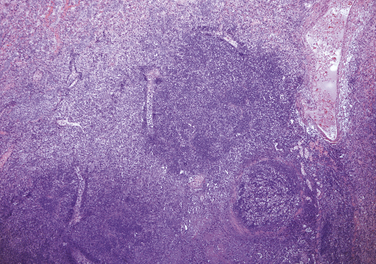
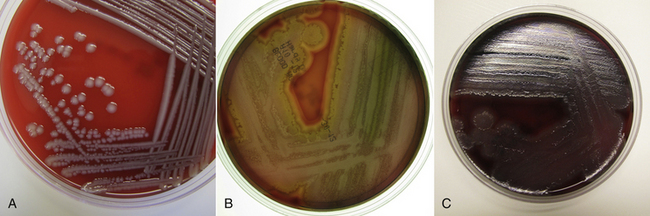
Chapter 36 Gram-negative bacteria are widespread causes of opportunistic infections in dogs and cats and primarily cause disease when host defenses are impaired. They are important causes of nosocomial disease. In contrast to gram-positive bacteria, gram-negative bacteria have a complex outer membrane that contains lipopolysaccharide (LPS), as well as structures known as porins that regulate transport of molecules in and out of the cell, which includes antimicrobial drugs. Between this outer membrane and the inner (cytoplasmic) membrane is a periplasmic space, which contains the structural polymer peptidoglycan (or murein) (Figure 36-1). The peptidoglycan layer of gram-negative bacteria is much thinner than that of gram-positive bacteria, approximately one layer, or 7 to 8 nm thick (compared with many layers, or 20 to 80 nm thick for gram-positive bacteria). The inner cytoplasmic membrane contains many different proteins that are lipoproteins anchored into the outer lipid bilayer leaflet, transmembrane proteins, or peripheral membrane proteins that lie adjacent to the bilayer leaflets. FIGURE 36-1 Structure of the envelope of gram-positive (A) and gram-negative (B) bacteria. The thickness of the peptidoglycan layer is greater in gram-positive bacteria than in gram-negative bacteria. LPS is a large molecule that varies in composition from one bacterial species and strain to another. It contributes to the structural integrity of gram-negative bacteria and is a potent virulence factor. It consists of the lipid A backbone, a core oligosaccharide, and the O antigen side chain. Lipid A is a phosphorylated disaccharide to which long, hydrophobic fatty acid chains are attached, which anchor the LPS into the outer membrane (Figures 36-1 and 36-2). The lipid A component is also known as endotoxin because it is the biologically active portion of the molecule, in that it stimulates a potent host inflammatory response (see Chapter 86). The core oligosaccharide connects lipid A to the O antigen side chain. Its composition differs between bacterial species. The O antigen is a repeating polysaccharide that projects from the surface of the bacterial cell and varies in length from 1 to 60 repeats. It is the antigenic portion of the molecule and is responsible for serogroup classification of gram-negative bacteria. Gram-negative cocci, such as Moraxella or Neisseria, are not widely recognized as significant pathogens of dogs and cats. However, they are commensals of the oral cavity of dogs and cats, can cause bite wound infections in humans,2 and Neisseria canis was isolated in pure culture from a deep mandibular abscess from one dog.3 Gram-negative rods are classified into Enterobacteriaceae and non-Enterobacteriaceae species. Enterobacteriaceae that cause disease in dogs and cats include Escherichia coli, Proteus, Salmonella, Enterobacter, Citrobacter, Serratia, and Klebsiella. Non-Enterobacteriaceae species include the Pasteurellaceae (Pasteurella multocida), as well as Pseudomonas aeruginosa and Acinetobacter. Other gram-negative bacteria are coccobacilli or spiral-shaped organisms and include Bartonella, Bordetella, Campylobacter, Francisella, Helicobacter, and Brucella. Escherichia coli is the most common cause of urinary tract infections (UTIs) in dogs and cats and can also be isolated from dogs and cats with pyometra, prostatitis, peritonitis, cholangitis, cholecystitis, bacteremia and endocarditis, bronchopneumonia (such as that secondary to aspiration), and pyothorax. There is one report of necrotizing fasciitis in a dog associated with E. coli infection.4 Antibiotic resistance in E. coli isolates from dogs and cats is an increasing problem; 51% of 376 isolates collected from sick dogs and cats in the United States in 2005 showed resistance to at least one drug, and 29% of the 376 isolates were multiple drug resistant (MDR; defined as resistant to three or more classes of antimicrobial drugs).5 The prevalence of resistance in 395 isolates from healthy dogs and cats from Canada was lower, 19%.6 Extended-spectrum β-lactamase enzyme production has also been identified in E. coli isolates from dogs and cats in the United States,7 and a few isolates were found to express New Dehli metallo-β-lactamase (NDM-1), which hydrolyses carbapenems.8 Nosocomial infections with MDR E. coli have also been described in dogs and cats. In humans, strains of E. coli that cause UTIs differ from those that cause diarrhea, and there is some evidence that this may also be true for strains that cause UTIs versus pyometra in dogs and cats.9–11 Strains of E. coli that cause UTIs belong to the pathotype extraintestinal pathogenic E. coli (ExPEC). These strains possess a number of virulence factors, such as siderophores, type 1 and P fimbriae, hemolysin, and cytotoxic necrotizing factor. Specific virulence factors, as well as biofilm-forming capacity, have been associated with persistent or relapsing UTIs,12,13 and uropathogenic E. coli can invade and persist within bladder epithelial cells. In one study, canine UTIs associated with E. coli were more likely to be recurrent than those associated with other uropathogens.14 There has been concern that dogs may act as a source of E. coli strains that are pathogenic for humans.11,15 In other extraintestinal E. coli infections, E. coli more closely resemble commensal strains, and host factors may be more important than bacterial virulence factors. The reader is referred to Chapter 46 for more information on intestinal E. coli infections. The most common species of Klebsiella that infects dogs and cats is K. pneumoniae. K. oxytoca is a nosocomial pathogen in humans and has been reported in association with intravenous catheter colonization in puppies with parvoviral enteritis.16 K. pneumoniae primarily causes upper and lower UTIs in dogs and cats, but can also cause pneumonia, sometimes with abscessation (Figures 36-3 and 36-4). It has also been isolated from dogs with hepatic abscesses.17 Nosocomial infections may be associated with bacteremia, endocarditis, and postoperative skin and soft tissue infections. The capsule of K. pneumoniae is a major virulence factor; it inhibits phagocytosis and is responsible for the mucoid appearance of the organism in culture (Figure 36-5, A). FIGURE 36-3 Lungs of an 11-year-old intact male German shepherd dog with severe, acute, suppurative, necrotizing and hemorrhagic bronchopneumonia due to Klebsiella pneumoniae infection. The lung lobes are severely congested. Five days before death, multiple laminectomies had been performed for intervertebral disc disease, and 2 days later the dog developed aspiration pneumonia that necessitated mechanical ventilation. Euthanasia was elected when there was further deterioration in the dog’s condition, and bloody exudate was present in the endotracheal tube just before euthanasia. FIGURE 36-4 Histopathology of the lung from a 4-month-old intact male Newfoundland dog with severe, multifocal, necrotizing lung abscesses. Intralesional rod-shaped bacteria were also present. Klebsiella pneumoniae was isolated from the lesions.H&E stain, 40× magnification. FIGURE 36-5 Gram-negative aerobic bacteria growing on blood agar.A, Mucoid colonies of Klebsiella pneumoniae. B, Pseudomonas aeruginosa. Transillumination using a light box revealed the presence of hemolysis and production of a green pigment (pyoverdin). C, The plate in (B) had a fruity odor and a “mother-of-pearl” appearance when not transilluminated. Serratia marcescens is an important cause of nosocomial infections in both human and veterinary medicine. In human patients it is often linked to intravenous drug use. The organism has a tremendous ability to survive in the environment and may contaminate and remain viable in disinfectant solutions.18 It sometimes grows as a contaminant, such as in blood cultures. Some, but not all, strains of S. marcescens produce a red pigment when grown in culture. In dogs, S. marcescens infection has been associated with UTIs, bacteremia and aortic valve endocarditis,19 catheter-related infections in canine parvoviral enteritis,14 and necrotizing fasciitis.20 Contamination of blood products was linked to an outbreak of bacteremia in cats.21 Serratia is resistant to ampicillin and first-generation cephalosporins as a result of a chromosomal β-lactamase. It may also acquire plasmid-mediated resistance to other antimicrobial drugs. Enterobacter cloacae and, to a lesser extent, Enterobacter aerogenes represent the majority of Enterobacter species isolated from dogs and cats in the author’s hospital. Enterobacter spp. are mainly isolated from dogs and cats with UTIs but occasionally cause wound or catheter-related infections. They generally are intrinsically resistant to ampicillin and first-generation cephalosporins and may also be resistant to multiple other antimicrobial drug classes. Citrobacter species are named for their ability to use citrate as a sole carbon source. Citrobacter freundii is most commonly isolated from dogs and cats with UTIs but can also cause catheter-related infections, bacteremia, and endocarditis.22,23 Citrobacter koseri was isolated from puppies with myocarditis.24 Like Enterobacter and Serratia, Citrobacter are usually resistant to ampicillin and first-generation cephalosporins and may acquire a number of plasmid-mediated resistance genes, including those that encode ESBLs and resistance to fluoroquinolones. Bacteria that belong to the family Pasteurellaceae are small, fastidious, nonmotile bacilli or coccobacilli that are commensals of the oral cavities and upper respiratory tracts of dogs and especially cats. The most common species of Pasteurella associated with disease in dogs and especially cats is Pasteurella multocida. Other species include Pasteurella canis, Pasteurella dagmatis, and Pasteurella stomatis. Pasteurella spp. have been associated with a wide variety of disease manifestations that include pyothorax, upper respiratory tract infections (usually secondary to viral infections or chronic rhinosinusitis), bronchopneumonia, UTIs, ocular surface infections, wound infections, cutaneous abscesses, otitis externa, bacteremia, and, rarely, infective endocarditis. They may be found in mixed infections with other bacteria, such as Streptococcus canis, other gram-negative bacteria, or anaerobes. In humans, they are an important cause of animal bite wound infections.2 Pasteurella spp. are usually broadly susceptible to antimicrobial drugs, and the treatment of choice is a penicillin such as penicillin G, ampicillin, or amoxicillin.
Gram-negative Bacterial Infections
Etiology, Epidemiology, and Clinical Features
Enterobacteriaceae
Escherichia coli
Klebsiella
Serratia marcescens
Enterobacter
Citrobacter
Non-Enterobacteriaceae
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Gram-negative Bacterial Infections
Only gold members can continue reading. Log In or Register to continue