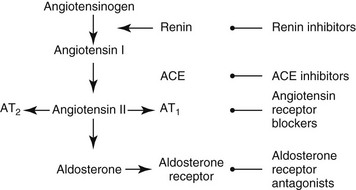
Chapter 188 Glomerular diseases frequently are associated with a specific set of clinicopathologic findings. Proteinuria (i.e., a urine protein/creatinine [UP/C] ratio >0.5) is the hallmark of glomerular injury. Certain glomerular disease histologic subtypes are associated with greater amounts of urine protein loss and higher UP/C ratios in people, but similar associations in dogs or cats with glomerular disease have been demonstrated inconsistently (Cook and Cowgill, 1996; Klosterman et al, 2011). Human patients with UP/C values of more than 2.0 to 3.5 or more than 300 to 350 mg/mmol are referred to as having nephrotic-range proteinuria. However, ranges of UP/C values noted in dogs with glomerular disease with and without nephrotic syndrome overlap so that identification of a similar clinically useful value has not been possible (Klosterman et al, 2011). If injury to renal tubules develops at a slower rate than does the glomerular injury, some renal concentrating ability may persist despite development of azotemia. In a subset of dogs and cats with glomerular disease, hypoalbuminemia and proteinuria are accompanied by hypercholesterolemia and extravascular fluid accumulation, referred to as nephrotic syndrome. Renal biopsy is required to determine the specific histologic subtype of glomerular disease. However, this test may not be indicated in every patient, and more study is needed to determine which patients may benefit from renal biopsy evaluation. Animals with International Renal Interest Society (IRIS) stage 1 or 2 (and possibly stage 3) chronic kidney disease that have UP/C values in or nearing the nephrotic range suggested in people likely will benefit most (see Chapter 189). Once collected, renal biopsy specimens should be divided appropriately and placed in fixatives suitable for evaluation with light, electron, and immunofluorescent microscopy. Although the findings of light microscopy may be highly suggestive of a particular glomerular disease, electron microscopy often is required for definitive characterization of histologic subtype, and immunofluorescent microscopy is needed to determine the specific nature of immune deposits. Intraglomerular hydrostatic pressure is a primary determinant of net protein movement across the glomerular filtration barrier. Angiotensin-converting enzyme (ACE) inhibitors, angiotensin II type 1 receptor blockers (ARBs), and aldosterone receptor antagonists inhibit the renin-angiotensin-aldosterone system (RAAS) and reduce glomerular capillary bed hydrostatic pressure, thus reducing proteinuria (Figure 188-1). Although the precise mechanisms by which these agents exert their beneficial effects have not been fully elucidated, it appears that reduction in proteinuria is greater than would be expected from their antihypertensive effects alone. Figure 188-1 Renin-angiotensin-aldosterone system and the sites of action of inhibitors used to reduce the severity of proteinuria in human and veterinary medicine. ACE inhibitors preferentially decrease efferent glomerular arteriolar resistance, which leads to decreased or normalized glomerular transcapillary hydrostatic pressure and also may preserve renal function by additional, less well-described mechanisms. Treatment of dogs with glomerular diseases with enalapril significantly reduces proteinuria and delays onset or progression of azotemia and therefore is considered a standard of care (Grauer et al, 2000). ACE inhibitors are administered once or twice daily; if the magnitude of azotemia is relatively stable and there has been only partial or no reduction in proteinuria after 4 to 8 weeks, the dosage may be increased to the upper end of the recommended ranges (e.g., enalapril or benazepril 2 mg/kg PO per day). Although serum creatinine concentration should be monitored after therapy is begun, it is uncommon for dogs and cats to become azotemic or to experience severe worsening of preexisting azotemia (i.e., >30% increase from baseline) as a result of ACE inhibitor administration alone. Although differences exist in the elimination pharmacokinetics of enalapril and benazepril, there is no evidence that one ACE inhibitor is pharmacokinetically superior to others in either dogs or cats. In people, up-titration of doses of ACE inhibitors beyond current recommendations maximizes reduction of proteinuria and recently has been suggested to improve prognosis (Schjoedt et al, 2009); similar studies have not been performed yet in dogs or cats. Although ARBs are used commonly in people with glomerular disease, their use in dogs still is being developed. Losartan is the most commonly used ARB. Even though dogs do not produce one of the major active metabolites, losartan does have anti-RAAS pharmacodynamic effects and anecdotally has an antiproteinuric effect in dogs with glomerular disease. In theory there may be an added benefit to combined therapy with an ACE inhibitor and an ARB because monotherapy with either drug class likely does not provide complete RAAS blockade. Studies in people have suggested that these drugs may have additive or synergistic effects in reducing proteinuria, although there have not been any equivalent studies in dogs (Linas, 2008). In addition, because the dose of each individual drug can be reduced during combined therapy, adverse effects in people may be reduced; however, elderly patients prescribed this combination have a higher risk of kidney failure and death. Serum aldosterone concentrations increase over time (known as aldosterone escape) in people treated with maximal dosages of ACE inhibitors or ARBs, which may have adverse effects on the heart, systemic blood vessels, and glomeruli (Bakris, 2008). Aldosterone receptor antagonists reduce proteinuria and stabilize kidney function, acting in an additive fashion with ACE inhibitors and ARBs in people. Spironolactone (1.0 to 2.0 mg/kg q12h PO) is used most commonly in veterinary medicine; however, there are no published data examining its usefulness in the treatment of dogs or cats with glomerular disease. This drug likely would be most effective in animals with high serum aldosterone concentrations and persistent proteinuria despite treatment with an ACE inhibitor, ARB, or both. Anecdotal experience suggests that spironolactone is not very effective in reducing proteinuria in dogs with glomerular disease. Treatment with antihypertensives often leads to a reduction in the magnitude of proteinuria in patients with high blood pressure. ACE inhibitors and ARBs are relatively weak antihypertensive agents and do not routinely reduce blood pressure by more than 10 to 15 mm Hg; additional antihypertensive agents (e.g., amlodipine) may be required if hypertension persists. Treatment with amlodipine alone also may result in reduction of proteinuria, but typically to a lesser degree than that seen with ACE inhibitor or ARB therapy. In addition, amlodipine may induce preferential dilation of the afferent glomerular arteriole, thereby increasing intraglomerular hydrostatic pressure, as well as RAAS activation in dogs and potentially also cats (Atkins et al, 2007). Some clinicians recommend that amlodipine not be administered to a dog or cat with chronic kidney disease without simultaneous administration of an ACE inhibitor.
Glomerular Disease and Nephrotic Syndrome
Diagnosis of Glomerular Disease
Nonspecific Management of Glomerular Disease
Reduction of Proteinuria
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


