Mads F. Bertelsen Two species exist within the artiodactylid family of Giraffidae; the giraffe (Giraffa camelopardalis) and the okapi (Okapia johnstoni). Giraffids first arose eight million years ago during the Miocene period, and fossil evidence suggests that the family was once much more extensive, with over 10 fossil genera described. Up to nine races or subspecies of giraffe have been described, although genetic research and the fact that distinct morphologic distinctions between groupings exist despite the lack of physical boundaries have led some authorities to consider several distinct species. The subspecies most commonly held by zoos are the reticulated giraffe (Giraffa camelopardalis reticulata), the Rothschild giraffe (G.c. rothschildi), and the Masai giraffe (G.c. tippelskirchi). Once widespread across the African continent, giraffes are now largely confined to national parks and game farms in eastern and southern Africa, with scattered populations in west Africa. Because of its height, the giraffe has access to browse unavailable to other species and thus may coexist with grazers and smaller browsers and even livestock. Adult giraffes are rarely preyed upon by predators, but calf mortality is high. Giraffes are sociable animals usually found in dynamic, ever-changing groups, the most stable of which are those composed by mothers and their young. Subadult males are social, whereas mature males become more solitary. With an estimated 80,000 animals left in the wild, the giraffe is classified by the International Union for the Conservation of Nature (IUCN) as a species “Of Least Concern.” However, several of the subspecies are now considered “Endangered” (e.g., West African and Rothschild). At least 2000 giraffes are maintained in captivity, making the captive population self-sustaining. Discovered by science only as recently as 1901, the okapi was the last large African mammal species to be described. The okapi is now endemic to the Democratic Republic of the Congo in central Africa, where they inhabit dense damp forests on both sides of the Congo River. Okapis are diurnal and live alone, in pairs, or in small family groups, but relatively little is known about their social structure, largely because of their remote habitat and timid nature. Estimated remaining wild population is between 10,000 and 35,000, and the fate of these animals is closely linked to the unstable political climate of the region. The okapi is listed as “Near Threatened” by the IUCN but is not listed on the Convention on International Trade in Endangered Species (CITES) Appendix. With less than 200 animals in captivity, the okapi population is considered fragile. With a height of up to more than 5 meters (m), giraffes are characterized by their extremely long necks and long legs, with considerably longer forelimbs than hindlimbs. The head is fairly small, with two horns or ossicones and a central osseous protuberance, which is particularly developed in the males. The tongue is long and flexible, its distal 20 cm pigmented. Mature males weigh 850 to 1950 kilograms (kg) and females 700 to 1200 kg. The internal anatomy of giraffes is analogous to that of the domestic cow and other artiodactylids. The dental formula for giraffes is incisors (I) 0/3, canines (C) 0/1, premolars (P) 3/3, molars (M) 3/3, for a total of 33. Often, the gallbladder is absent, although it occurs in some individuals. Two jugular veins run immediately under the skin on either side of the ventral neck. The skin varies in thickness from being thin on the ears and medial aspects of the legs to being thick along the neck and lateral body. The thick skin aids in edema prevention in the lower leg and forms a dermal armor for protection against predators or fighting with conspecifics. The dark patches of the skin have been suggested to have a thermoregulatory role in acting as regions where heat loss to the environment is enabled by selective vasodilation. The relative lung mass as well as volume is only approximately 60% of that of other mammals, and the lung volume-to-body mass ratio decreases during growth.43 The extremely long trachea has a diameter significantly narrower than in similar-sized mammals, so the dead space volume, although greater than in most species, is not as large as could be expected and is compensated for by a slightly larger tidal volume.43 Previously described as extraordinarily large, the giraffe heart has a relative weight of approximately. 0.5% of body weight, which is essentially identical to that of other mammals.42 The basis for the massive blood pressure generated is smaller ventricular radii and an unusually thick left ventricular wall with oblique muscle fibers. The giraffe vein has a venous valve layout similar to that in other large mammals, and no arterial valves exist.49 The okapi reaches a head-to-body length of 200 to 210 cm and a shoulder height of 150 to 170 cm and has a body weight of 200–300 kg. Females are larger than males. The eyes and ears are large, and the tongue long enough to reach the ear base. Males have a pair of short-haired ossicones that are directed backward. The body is short and compact with a sloping back, as in the giraffe, but the neck is much shorter. Available information on okapi anatomy and physiology is limited, but the dental formula and internal anatomy resemble those of the giraffe. To compensate for the hydrostatic challenge of perfusing the brain, the giraffe heart generates a blood pressure twice that of other mammals, and its cardiovascular anatomy and physiology have been subject to considerable speculation and myths. Both stroke volume and cardiac output are lower than in similar-sized mammals. Blood volume is unusually low, and compliance of the vascular system is also low. The peculiar vascular anatomy—with narrow, rigid veins with low compliance in the legs and large, compliant veins in the neck region—gives rise to an interesting and nonintuitive physiologic phenomenon.6 When the head of the anesthetized giraffe is lowered, blood pressure at head-level briefly spikes, before returning to much lower values. The lowering of the blood pressure coincides with pooling of blood in the compliant jugular veins, giving rise to a decreased cardiac preload and consequently lower systemic blood pressure (Frank-Starling mechanism). As a consequence of this mechanism, the arterial pressure at head level is maintained at or near 100 millimeters of mercury (mm Hg), and the central blood pressure is directly proportional to the position of the head relative to the heart. Because of the high arterial pressures and the hydrostatic pressure, the arterial pressure in the lower leg may exceed 450 mm Hg. Edema in this region is prevented through a gravity-suit-like fascia and skin, prominent lymphatics, and well-developed valves in veins and lymphatics, as well as an abrupt narrowing of the arterial lumen at the level of the elbow or stifle. The giraffe kidney experiences much higher pressures compared with the human kidney and appears to cope with this through a fibrous capsule and an increased interstitial pressure of about 30 to 40 mm Hg. This means that normal kidney perfusion depends on a mean arterial pressure of at least 130 mm Hg. Similar to camels, the giraffe is capable of varying the body temperature within a couple of degrees Celsius, saving energy otherwise needed for increasing the temperature at night and cooling during daytime. Giraffes may learn to lower their heads to walk through doors only slightly higher than their withers; however, stressed or sedated animals will often not do this, which necessitates high doors for a giraffe house. Soft flooring and lack of exercise may lead to overgrowth of feet and the need for trimming, so the giraffe should be encouraged to walk on abrasive surfaces. Coarse gravel may be used on top of concrete to provide traction and wear. Neonates require sure footing and do best when born on pasture or a thick layer of bedding to prevent splaying. Giraffes have a high surface-to-volume ratio and are adapted to tropical climates. In moderate climates, they may be maintained in outdoor enclosures year-round. In temperate climates, access to stables heated to the range of 18° C to 24° C (65° F–75° F) must be provided, and in subzero temperatures, outdoor access should be restricted. Both okapis and giraffes are prone to sterotypies, particularly those involving the tongue, and it is important to incorporate in enclosure design pulleys and other systems to provide browse and enrichment items at head level. When designing facilities for giraffids, the logistics of loading and unloading animals should also be considered. Ideally, narrow walkways leading to an appropriate docking ramp for transport vehicles should be incorporated into the design. Both giraffes and okapis are selective browsers seeking out the high-nutrient components of plants such as fresh leaves and buds. In the wild, giraffes mainly feed on Acacia species, and the natural diet of the okapi includes a variety of species. In an attempt to avoid negative energy balance, captive diets have traditionally contained high levels of protein (15%–20%) and starch (20%–30%). Based on wild diets, current recommendations include crude protein levels of only 10%–14%, starch levels below 5%, fat 2%–5%, and high amounts of fiber (minimally 25% acid detergent fiber) all based on a dry-matter basis.58 Care should be taken to keep calcium levels high and phosphorus levels low. The new diet regimens have recently been shown to lead to increased serum levels of magnesium as well as n3 and n6 fatty acids and decreased levels of phosphorus and saturated fatty acids36,41 so that blood nutrient profiles more closely match those of free-ranging giraffe.36 The importance of browse, for both the nutritional value and the behavioral well-being of animals, cannot be overstated and browse should be provided to the greatest extent possible, but good-quality hay and alfalfa as well as silage may be substituted. Surplus buds and twigs from rose growers have been used successfully in okapis.59 The precise mineral and vitamin requirements of giraffids have not been established, but animals should have access to trace mineral salt blocks, and copper supplementation should be considered if deficiencies are suspected. Giraffes may be quite tame and may become habituated to some manipulation, including blood sample collection and light hoof trimming; however, many individuals do not respond well to this approach. The safest nonchemical method for collecting routine samples and closely examining animals is to accustom them to a chute during daily routines. Forced physical restraint without specialized stalls or chutes is likely to be unsuccessful and dangerous. Giraffes may deliver a formidable kick with all four legs in essentially any direction. In tall narrow chutes, with secure footing for personnel as well as animals, giraffes may be physically restrained for minor procedures such as injections, blood collections, tuberculosis testing and so on, but the risks involved for the animal as well as the staff should be kept in mind. Okapis respond well to training and positive reinforcement and poorly to physical restraint. Once they get started, giraffes have the tendency to keep walking along hallways and so on, which may be exploited for crating or loading into vehicles. A curtain with a weight at the bottom, which will fall from a horizontal position to a vertical position behind the animal when released, may be helpful to encourage the animal to take that last step into an unknown crate. Giraffe anesthesia remains a challenge because of considerations of size as well as the peculiar anatomy and physiology of these animals; however, several good protocols and excellent information resources are now available.12 Standing sedation may work well, but ataxia may develop, so it is important to be prepared for the animal to go down unexpectedly. A chute or restraint device is ideal, but a large door that can close off a triangular space may provide a similar confined area. Several drug combinations have been used with success for procedures such as clinical examinations, hoof trimming, reproductive manipulation, minor surgery, and catheter placement (Table 61-1). TABLE 61-1 Protocols for Chemical Restraint Used in Giraffidae* * Refer to text for details. † Provide oxygen via deep nasal cannula or intubate. Two main schools in giraffe anesthesia exist: (1) opioid-based protocols7,11,66,67 and (2) ketamine, combined with high-dose medetetomidine.8,39 The latter approach has been popular over the past decade because of the avoidance of an opioid component and relatively smooth inductions; however, it may result in hypertension and tachypnea, and re-sedation from medetomidine as the reversal agent wears off is a real concern. The opioids, however, may induce excitation and hyperthermia if underdosed and result in hypoventilation when used in high dosages. A compromise, which involves incorporating opioids, ketamine, and α2-agonists in one protocol, appears to be the best solution so far.11,24 Refer to Table 61-1 for suggested protocols. The giraffe has traditionally been considered one of the most challenging animals to anesthetize, and most problems arise during induction and recovery. The key to success is careful planning and the availability of trained personnel and necessary equipment. The ideal induction occurs in a well-designed restraint device, which may be opened fully once the animal is recumbent. The second-best solution is a chute-system, where a halter may be placed on the sedated giraffe prior to induction, which will allow control of the head via a rope and pulley. The third-best option is to place a loop of thick rope around the base of the neck of the sedated animal and “walk” the animal to an open area with no obstacles, where it is made to walk in circles, with one to three handlers holding the rope. The animal is then tripped with another rope while still awake enough to maintain some control of the head during the fall. If neither of these options are available, the animal may be allowed to become recumbent in a padded stall or at least in an area without major obstacles. In either case, it is crucial to gain control of the animal’s head as soon as it becomes recumbent, as most injuries occur when the heavily sedated giraffe attempts to stand and falls again. For anesthetic induction in okapi, a padded restraint device is the safest option, but a quiet stall with sure footing will suffice. With opioid-based protocols, induction may sometimes result in excitement or tumbling. A staged approach, in which sedatives are allowed to set in for 15 to 20 minutes prior to opioid administration, is preferable, and once the opioid takes effect, two experienced helpers may use mild physical restraint with mattresses or boards to prevent injury.12 Regurgitation under anesthesia may be a concern in both giraffes and okapis, but particularly in the latter. The frequent early reports of regurgitation in okapis sedated with Immobilon (etorphine and acepromazine) was attributed to etorphine but likely largely was a reaction to acepromazine, which this author considers contraindicated in okapis. In animals fed mainly hay and pelleted feed, withholding food and water for 12 hours prior to a planned procedure is sufficient, but in animals eating large amounts of fresh browse, a 24-hour period is advisable. To minimize pulmonary compromise and ventilation–perfusion mismatch, a sternal position is preferable, when feasible, but giraffes generally do well in lateral recumbency for shorter periods. To reduce the risk of regurgitation and to stabilize blood pressure (see Unique Physiology section), the head should be elevated 80 to 150 cm above heart level. The neck should be kept as straight as possible, and placement of a padded board or ladder under the shoulder and onto bales of straw or similar material works well for this purpose. Performing endotracheal intubation is straightforward in giraffes. Direct visualization of the larynx is possible with the use of a long laryngoscope blade, and insertion of a relatively thin catheter to subsequently guide the endotracheal tube is a good option.12 However, in giraffes larger than 350 to 400 kg, the fastest approach is to manually insert a stomach tube or similar device into the trachea and then thread the endotracheal tube over that. Appropriate endotracheal tube sizes are 20 to 25 millimeters (mm) for okapis and juvenile giraffes and 25 to 30 mm for adult giraffes. Hypoventilation is often a concern, and oxygen should be provided, whenever possible. A Hudson demand valve or similar device will provide the animal with oxygen and allow intermittent ventilation, as needed. Even in animals breathing well, a “sigh” every 2 to 5 minutes appears to be beneficial to avoid alveolar closing and shunting. In animals not intubated, supplemental oxygen via a deep nasal cannula and flowing at one liter per 100 kilograms per minute is recommended. For recovery, a quiet area with good footing should be provided. Adequate space should be available for the animal to swing its head forward as it gets onto its hindfeet, and obstacles should be removed to avoid injury if the animal falls over. Reversal agents may be given intramuscularly or intravenously (IM or IV), depending on the situation. The goal is to get the animal into sternal recumbency, with strong spontaneous ventilation as fast as possible, and to then keep it there as long as possible, ideally for 10 to 15 minutes. Keeping the animal blindfolded during this time helps prevent its attempts to stand prematurely. Doxapram (0.1 mg/kg, given rapidly IV) may be used to stimulate animals that are reluctant to get up.24
Giraffidae
Biology15,47
Unique Anatomy15,47
Giraffe
Okapi
Unique Physiology6,29,42,43,49
Special Housing Requirements9
Feeding
Restraint and Handling
Chemical Restraint
Standing Sedation
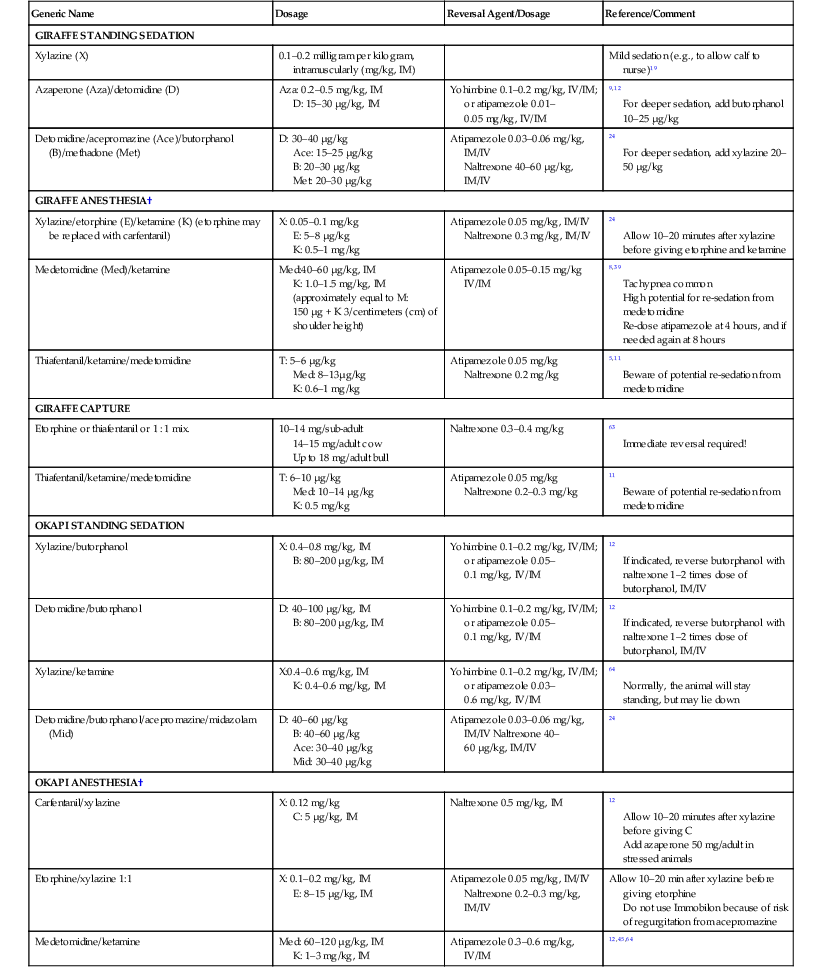
Generic Name
Dosage
Reversal Agent/Dosage
Reference/Comment
GIRAFFE STANDING SEDATION
Xylazine (X)
0.1–0.2 milligram per kilogram, intramuscularly (mg/kg, IM)
Mild sedation (e.g., to allow calf to nurse)19
Azaperone (Aza)/detomidine (D)
Aza: 0.2–0.5 mg/kg, IM
D: 15–30 µg/kg, IM
Yohimbine 0.1–0.2 mg/kg, IV/IM; or atipamezole 0.01–0.05 mg/kg, IV/IM
9,12
For deeper sedation, add butorphanol 10–25 µg/kg
Detomidine/acepromazine (Ace)/butorphanol (B)/methadone (Met)
D: 30–40 µg/kg
Ace: 15–25 µg/kg
B: 20–30 µg/kg
Met: 20–30 µg/kg
Atipamezole 0.03–0.06 mg/kg, IM/IV
Naltrexone 40–60 µg/kg, IM/IV
24
For deeper sedation, add xylazine 20–50 µg/kg
GIRAFFE ANESTHESIA†
Xylazine/etorphine (E)/ketamine (K) (etorphine may be replaced with carfentanil)
X: 0.05–0.1 mg/kg
E: 5–8 µg/kg
K: 0.5–1 mg/kg
Atipamezole 0.05 mg/kg, IM/IV
Naltrexone 0.3 mg/kg, IM/IV
24
Allow 10–20 minutes after xylazine before giving etorphine and ketamine
Medetomidine (Med)/ketamine
Med:40–60 µg/kg, IM
K: 1.0–1.5 mg/kg, IM
(approximately equal to M: 150 µg + K 3/centimeters (cm) of shoulder height)
Atipamezole 0.05–0.15 mg/kg IV/IM
8,39
Tachypnea common
High potential for re-sedation from medetomidine
Re-dose atipamezole at 4 hours, and if needed again at 8 hours
Thiafentanil/ketamine/medetomidine
T: 5–6 µg/kg
Med: 8–13µg/kg
K: 0.6–1 mg/kg
Atipamezole 0.05 mg/kg
Naltrexone 0.2 mg/kg
5,11
Beware of potential re-sedation from medetomidine
GIRAFFE CAPTURE
Etorphine or thiafentanil or 1 : 1 mix.
10–14 mg/sub-adult
14–15 mg/adult cow
Up to 18 mg/adult bull
Naltrexone 0.3–0.4 mg/kg
63
Immediate reversal required!
Thiafentanil/ketamine/medetomidine
T: 6–10 µg/kg
Med: 10–14 µg/kg
K: 0.5 mg/kg
Atipamezole 0.05 mg/kg
Naltrexone 0.2–0.3 mg/kg
11
Beware of potential re-sedation from medetomidine
OKAPI STANDING SEDATION
Xylazine/butorphanol
X: 0.4–0.8 mg/kg, IM
B: 80–200 µg/kg, IM
Yohimbine 0.1–0.2 mg/kg, IV/IM; or atipamezole 0.05–0.1 mg/kg, IV/IM
12
If indicated, reverse butorphanol with naltrexone 1–2 times dose of butorphanol, IM/IV
Detomidine/butorphanol
D: 40–100 µg/kg, IM
B: 80–200 µg/kg, IM
Yohimbine 0.1–0.2 mg/kg, IV/IM; or atipamezole 0.05–0.1 mg/kg, IV/IM
12
If indicated, reverse butorphanol with naltrexone 1–2 times dose of butorphanol, IM/IV
Xylazine/ketamine
X:0.4–0.6 mg/kg, IM
K: 0.4–0.6 mg/kg, IM
Yohimbine 0.1–0.2 mg/kg, IV/IM; or atipamezole 0.03–0.6 mg/kg, IV/IM
64
Normally, the animal will stay standing, but may lie down
Detomidine/butorphanol/acepromazine/midazolam (Mid)
D: 40–60 µg/kg
B: 40–60 µg/kg
Ace: 30–40 µg/kg
Mid: 30–40 µg/kg
Atipamezole 0.03–0.06 mg/kg, IM/IV Naltrexone 40–60 µg/kg, IM/IV
24
OKAPI ANESTHESIA†
Carfentanil/xylazine
X: 0.12 mg/kg
C: 5 µg/kg, IM
Naltrexone 0.5 mg/kg, IM
12
Allow 10–20 minutes after xylazine before giving C
Add azaperone 50 mg/adult in stressed animals
Etorphine/xylazine 1:1
X: 0.1–0.2 mg/kg, IM
E: 8–15 µg/kg, IM
Atipamezole 0.05 mg/kg, IM/IV
Naltrexone 0.2–0.3 mg/kg, IM/IV
Allow 10–20 min after xylazine before giving etorphine
Do not use Immobilon because of risk of regurgitation from acepromazine
Medetomidine/ketamine
Med: 60–120 µg/kg, IM
K: 1–3 mg/kg, IM
Atipamezole 0.3–0.6 mg/kg, IV/IM
12,45,64
Immobilization and Anesthesia
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Giraffidae
Chapter 61
