Chapter 2 General Principles of Bacterial Disease Diagnosis
SPECIMEN SELECTION, COLLECTION, AND TRANSPORT
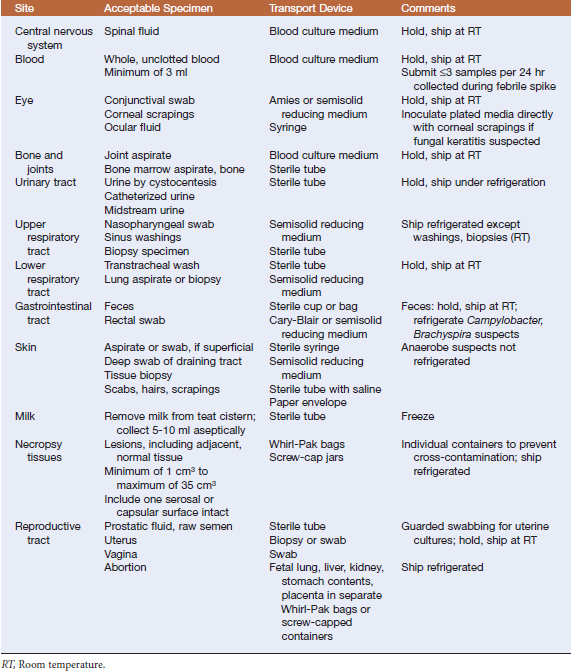
The first principle seems self-evident, but transgressions against it are common: specimens must be obtained aseptically from a site that is representative of the disease process. In some instances, the appropriate course is obvious; for example, urine culture is unlikely to yield clinically relevant information in a dog manifesting clinical signs of otitis externa. However, in some instances the choice of correct specimen maynot be quite so straightforward; a swab of nasal drainage might seem suitable for diagnosis of pneumonia, but in most cases a transtracheal wash is more appropriate (Table 2-1).
Specimens should be placed in appropriate transport devices to maintain a buffered andnonnutritive environment to prevent metabolic damage to organisms of interest and overgrowth of contaminants (Table 2-2). It is imperative to keep swabs moist to prevent bacterial desiccation and loss of viability. If samples are to be cultured aerobically, swabs should be placed in Stuart’s, Cary-Blair, or Amies medium for transport.They should not be placed in sterile containers with bacteriostatic saline solutions or ethylenediaminetetraacetic acid (EDTA), which is bactericidal. Aspirated specimens may be left in the syringe (with the needle removed), or may be placed into sterile tubes. Tissue specimens may be transported in sterile tubes or in Whirl-Pak bags. Fecal specimens should be collected in sterile cups or bags, but if Campylobacter spp. is suspected, feces should be placed in Cary-Blair transport medium.
TABLE 2-2 Specimen Transport Systems
RT, Room temperature.
Refrigeration usually preserves viability and reduces overgrowth by extraneous organisms, but fastidious organisms (such as anaerobes)will sometimes die rapidly when exposed tolow temperatures. Specimens likely to contain these organisms, or samples of body fluidsother than urine, should be held at room temperature.
Legible labeling of specimens, with an indelible marker, is critical. Note the source and/orspecific body site, and provide relevant clinical information on the submittal form to facilitate appropriate specimen handling and interpretation of results. Information regarding the animal species and anatomic site of origin, the suspected causative agent, previous antimicrobial therapy, and clinical signs will help the laboratory make the best choices regarding media and incubation conditions. Delivery to the laboratory should be within 48 hours of collection. Regulations governing the shipment of clinical specimens are changing rapidly, and compliance with prevailing laws may be facilitated by consulting the U.S. Department of Transportation (www.dot.gov).