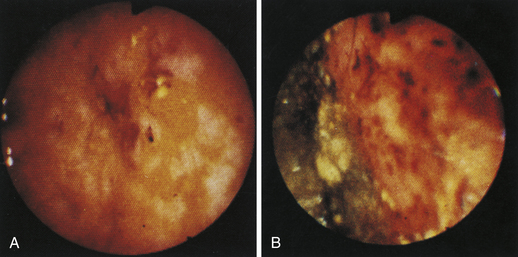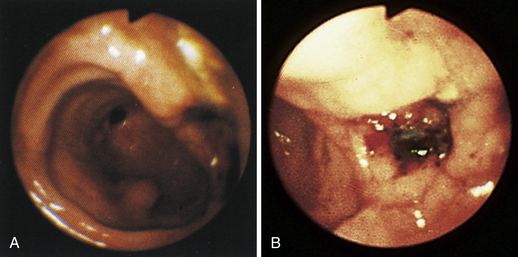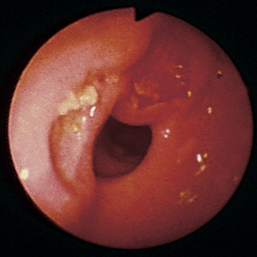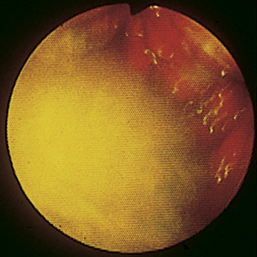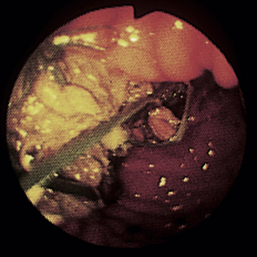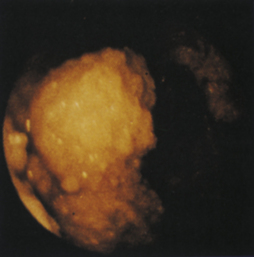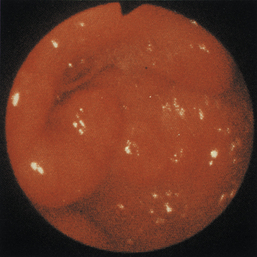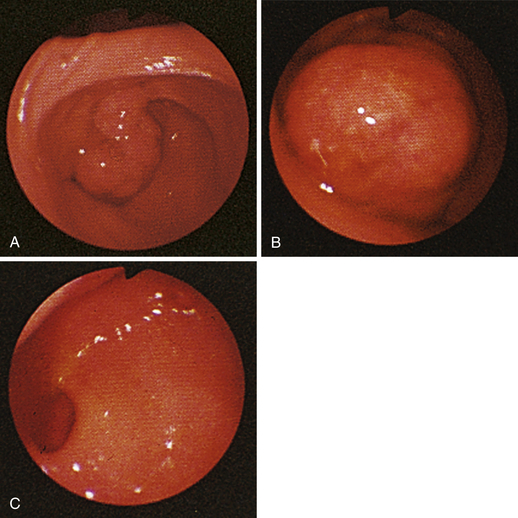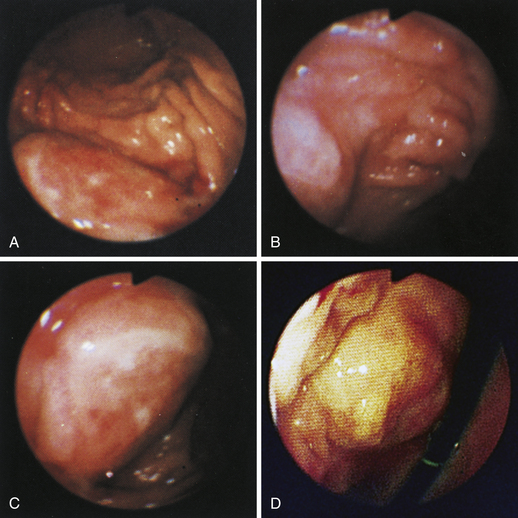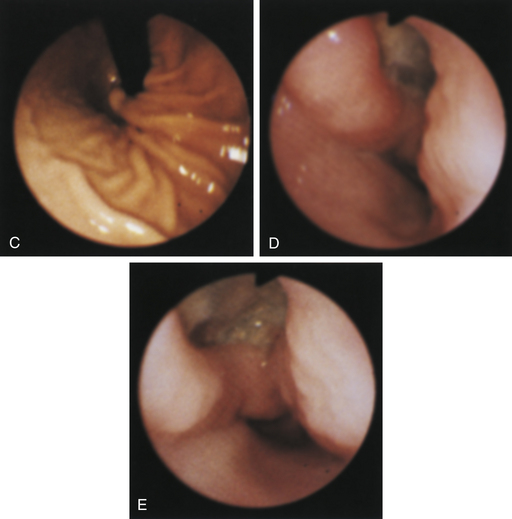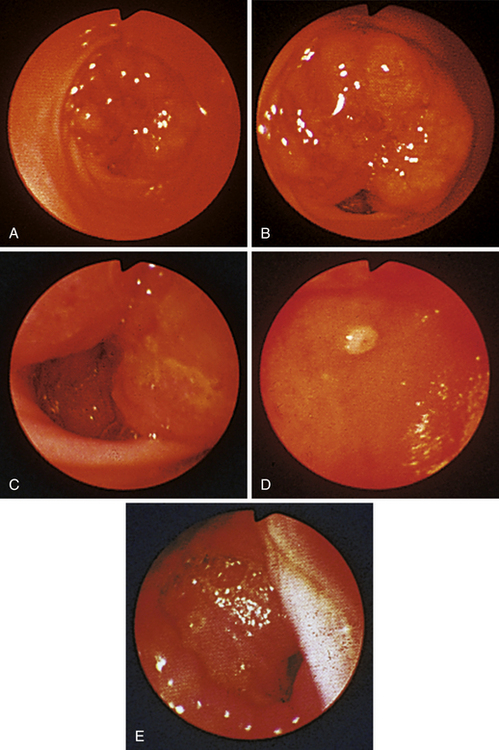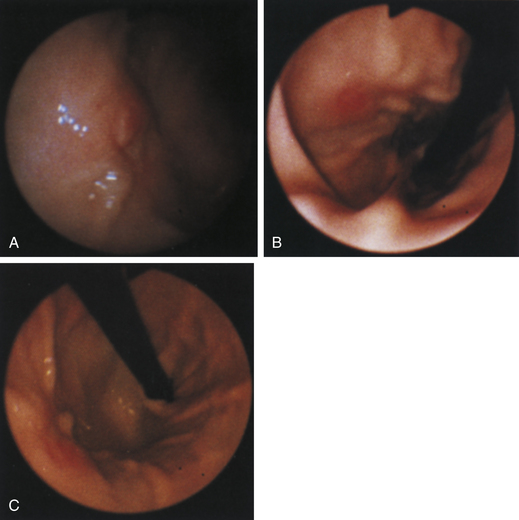
Figure 4-98 Endoscopic examination of the cat shown in Figure 4-97, done 19 days after the initial examination. Treatment involved only chemotherapy (prednisone, cyclophosphamide, and vincristine). A, Forward view of the proximal stomach at slightly more than moderate distension. The mass, which had decreased dramatically in size, is in the center of the field. (Compare with Figure 4-97, C.) B, Retroversion view. The site of the mass is barely detectable (focal reddened area to left of center). (Compare with Figure 4-97, D.) C, Retroversion view at a different angle. The mass can be seen at the 7-o’clock position.
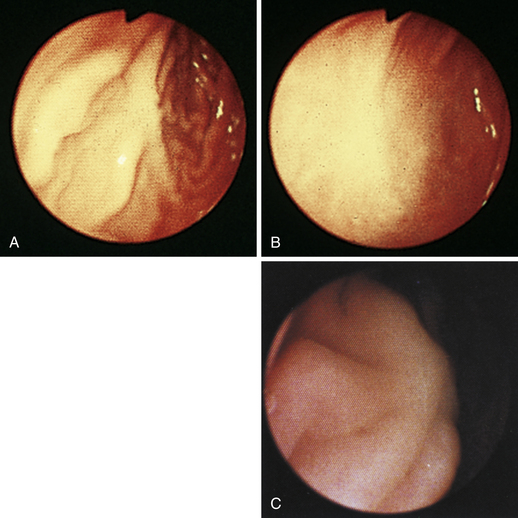
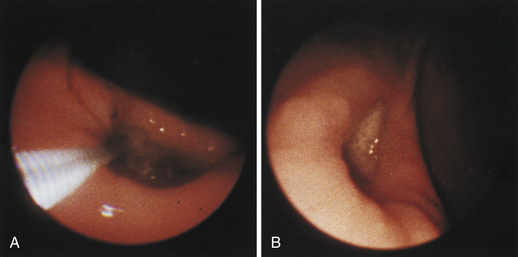
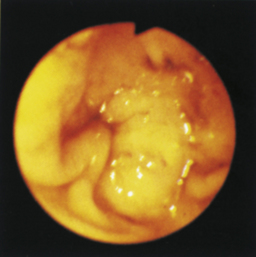
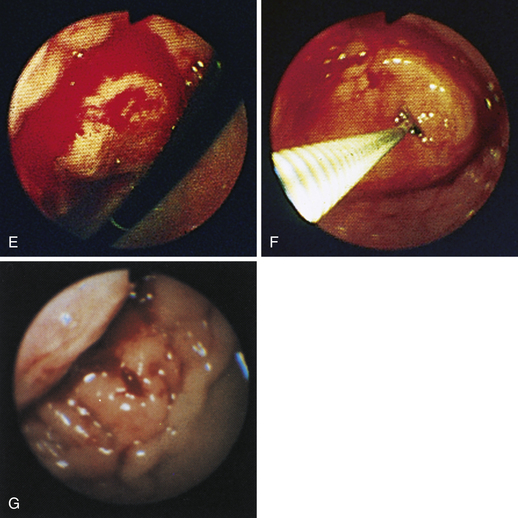
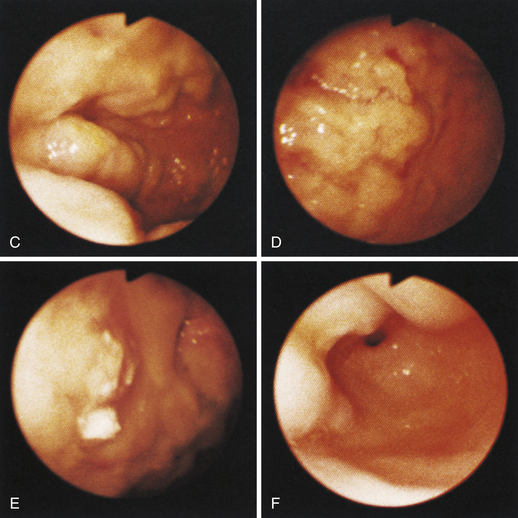
Figure 4-99 Endoscopic photographs of the cat discussed in Figures 4-97 and 4-98, obtained 19 weeks after the initial examination. The patient was doing extremely well while undergoing chemotherapy. No evidence of a gastric mass is seen, and the mucosa at the original site of the lesion appears whiter than the surrounding mucosa. A, Forward view of the proximal stomach in mild distension. Note that the rugal folds no longer appear thickened or irregular. B, Same site as in A but with moderate distension. C, Close-up view of the gastric mucosa. Note the smooth mucosal surface.
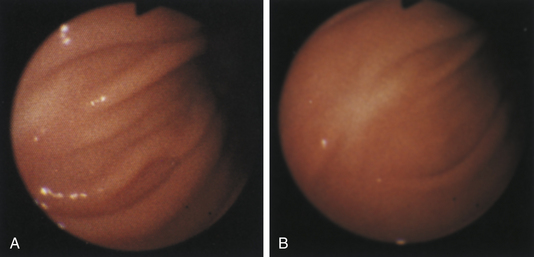
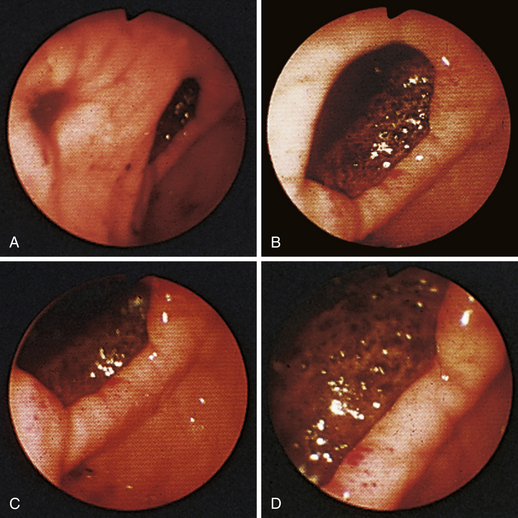
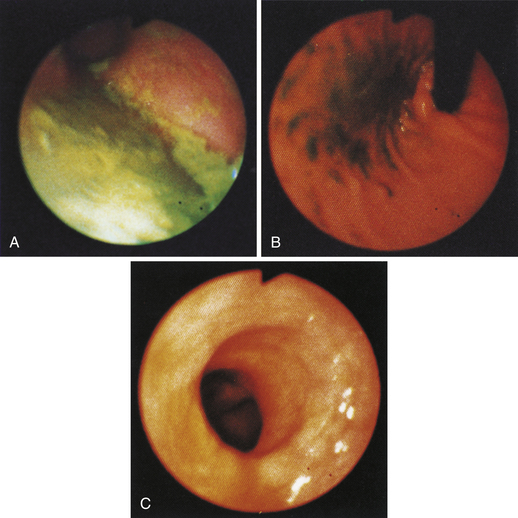
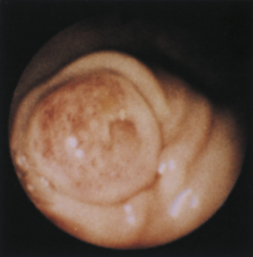
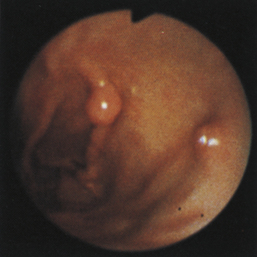
Figure 4-100 Endoscopic photographs of the cat in Figures 4-97, 4-98, and 4-99, taken 9 months after the animal was first examined. A, Forward view of the proximal stomach in mild distension. B, Forward view of the stomach in moderate distension. The stomach is grossly normal except for the small, whitened area where the mass was previously located (compare with Figures 4-97, C; 4-98, A; and 4-99, A). Biopsy samples showed no evidence of lymphosarcoma. The cat was still receiving chemotherapy and was clinically normal. Chemotherapy was discontinued at 12 months; at 18 months, no evidence of tumor recurrence was found. Note: The cat lived 8 years after the diagnosis was made. Chemotherapy never had to be resumed. This case highlights the importance of using endoscopy relatively early in animals with unexplained chronic vomiting. This cat’s history was easily consistent with a diagnosis of inflammatory bowel disease or chronic gastritis, yet endoscopy revealed lymphosarcoma. Early initiation of the most indicated therapy resulted in an excellent outcome. (Also see Figure 4-110 for illustration of an additional incident involving this patient: gastric hairball.)
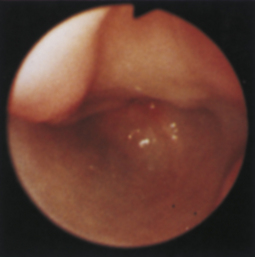
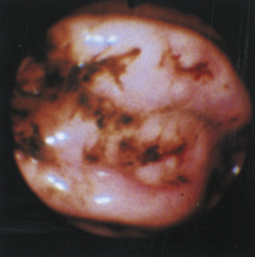
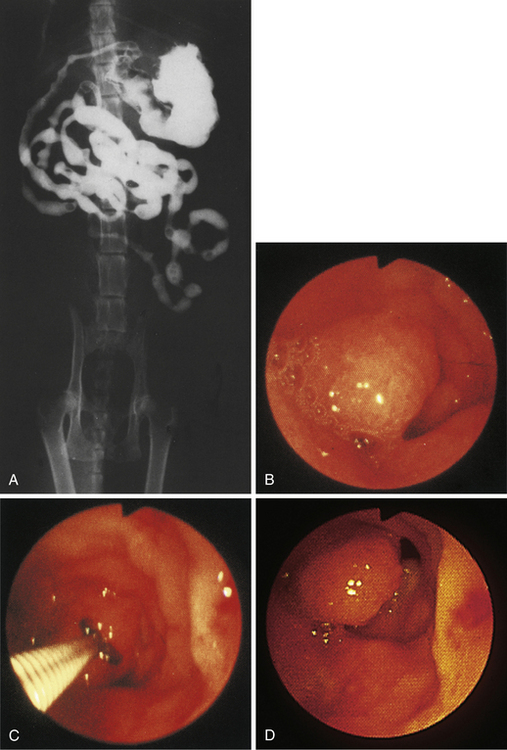
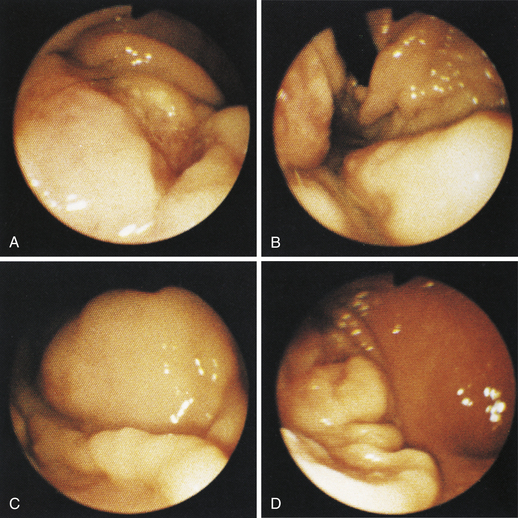
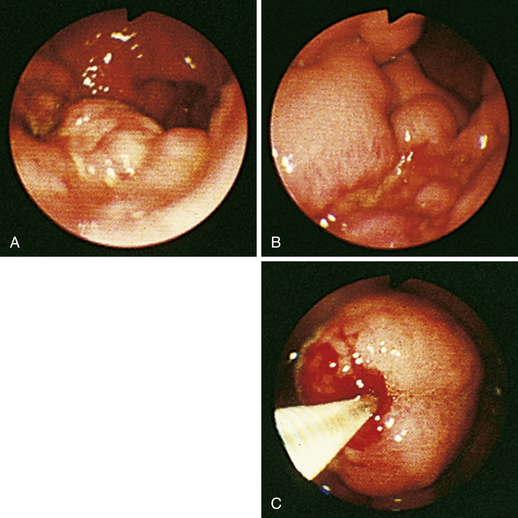
Figure 4-104 Examination of the cat shown in Figure 4-103, performed 5 weeks after presentation. Chemotherapy dramatically decreased the size of the antral masses. The endoscope is in the same position as in Figure 4-103, D-E. The pylorus is closed, but its orifice is seen in the center of the field. One of the antral masses, although somewhat smaller, is still evident in the left aspect of the field. The debris-filled cavern is no longer present.
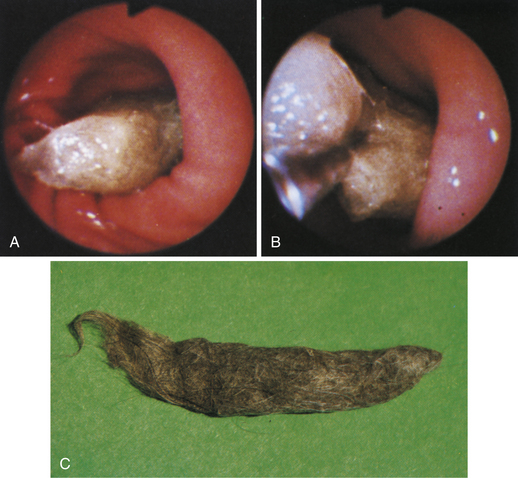
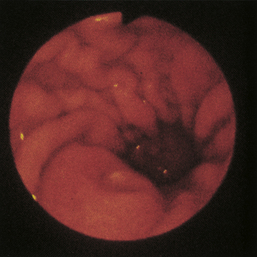
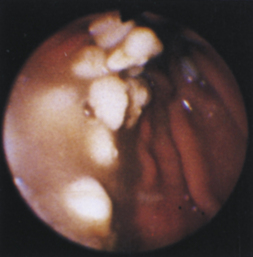
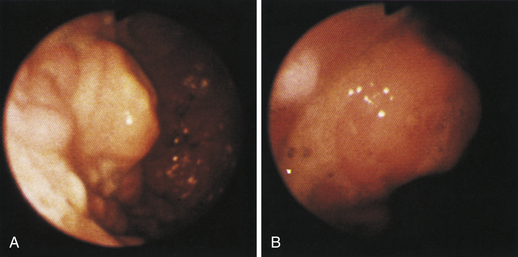
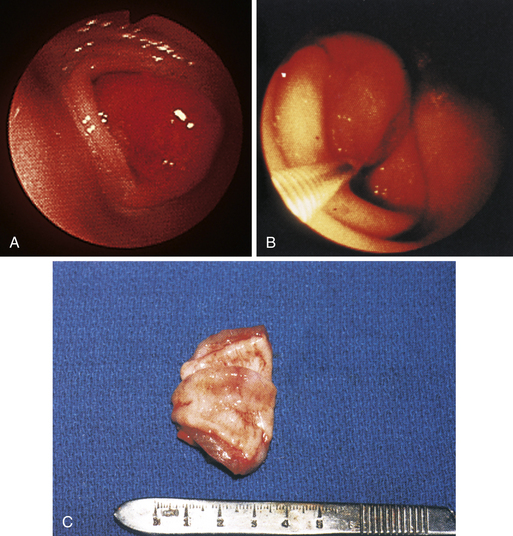
Figure 4-110 A, A large tubular hairball extends slightly into the distal gastric body from the antrum. The angulus is in the right aspect of the field. B, The hairball has been grasped with a two-prong foreign body grasper and is being pulled into the gastric body. C, The retrieved hairball. Note: These photographs are of the cat with gastric lymphosarcoma that was discussed in Figures 4-97 through 4-100. The owner reported recurrence of intermittent vomiting 19 weeks after the animal began receiving chemotherapy. Based on gastroscopy (no evidence of any mass recurrence) and the cessation of vomiting after the hairball removal, the vomiting was caused by the hairball and not loss of remission from lymphosarcoma.
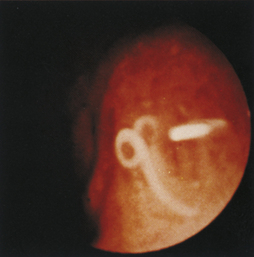
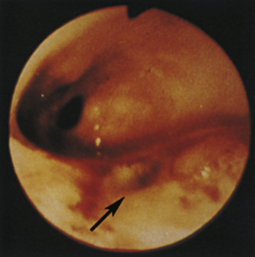
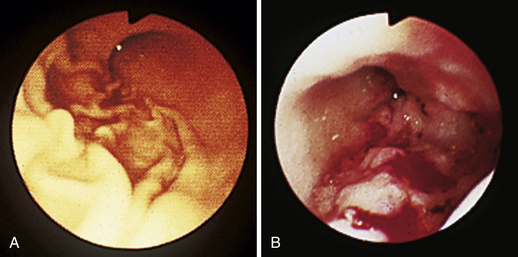
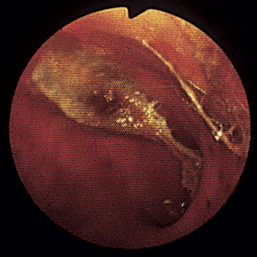
Figure 4-111 Physaloptera parasite on the gastric mucosa of a dog (center of field). The white image at the right (3-o’clock position) is due to light reflection. See also Figure 8-18 for another example of Physaloptera parasites observed at gastroscopy.
(Courtesy of Michael S. Leib.)
Chapter 4 Gastroscopy
Indications
An initial diagnostic plan for an animal with a chronic vomiting disorder should include a complete history, physical examination, complete blood count, complete biochemical profile (including thyroid evaluation for vomiting cats), urinalysis, fecal examination for parasites (both centrifugal flotation and a Giardia antigen test), evaluation for heartworm disease in cats (starting with a heartworm antibody test), and survey abdominal radiographs. Once disorders such as metabolic abnormalities (e.g., renal failure, diabetes mellitus, and liver disease), a foreign body that can be readily diagnosed on survey abdominal radiography, and dietary indiscretion or food sensitivity are ruled out, the decision to perform more in-depth diagnostic tests is made. This may include testing for disorders that may not be identified on baseline screening tests (e.g., consider the possibility of pancreatitis not diagnosed in the acute phase, leptospirosis, and atypical hypoadrenocorticism), barium contrast study, ultrasonography, or upper gastrointestinal (GI) endoscopy (or a combination of ultrasonography and gastroscopy). Clinical acumen is important in deciding which tests make the most sense, based on patient presentation and recent history and physical examination findings. Given the insensitivity of barium contrast studies for diagnosing mucosal disorders, the thoroughness of a complete gastric endoscopic examination combined with the ability to examine the upper small intestine at the same time, and the cost-containment factor that concerns many pet owners, the decision to choose gastroscopy over contrast radiography is usually a sound one. Ultrasonography is especially useful for evaluating wall thickness. The conclusion that endoscopy is an excellent way to examine for gastric disease should come as no surprise to the increasing number of small animal practitioners who are performing endoscopy. If radiographs identify a lesion or foreign body, gastroscopy or surgery is still necessary for definitive diagnosis and treatment. Furthermore, a normal gastric contrast radiographic examination does not rule out the presence of a gastric disorder.
Instrumentation
For small animal patients a complete evaluation of the stomach is best accomplished with the use of a flexible endoscope with a diameter of 9.8 mm or less and four-way tip deflection capability (see Chapter 2). In cats and in dogs weighing less than 5 kg (11 lb) an endoscope insertion-tube diameter of 9 mm or less is highly preferred (smaller 7.8-mm scopes are ideal). The use of a smaller endoscope makes it much more likely that the pyloric canal and proximal duodenum can be traversed and examined in these small patients than when a larger diameter scope is used, especially if the operator has limited experience.
Anesthesia and Positioning
Commonly used protocols for upper GI endoscopy include the use of acepromazine and butorphanol for premedication tranquilization/sedation, which will help calm patients before catheter placement and lower the induction and inhalant anesthetic dose requirements, thereby improving cardiovascular performance and easing recovery. Drugs that potentiate vomiting should be avoided in animals with esophageal or gastric foreign bodies (e.g., medetomidine or pure opioid agonists). The patient should be induced with an injectable anesthetic (propofol is most commonly used, but the combination of ketamine/diazepam is also acceptable) and intubated quickly. The endotracheal tube cuff should be appropriately inflated at all times so that inadvertent aspiration of fluid during the procedure is avoided. The cuff should not be deflated until the patient is extubated. Anesthesia is maintained with isoflurane or sevoflurane. Fluid support should be given throughout the procedure as needed (e.g., a balanced, isotonic crystalloid fluid such as Normosol-R or lactated Ringer’s solution administered at 10 mL/kg/hr for patients with normal oncotic pressure and plasma proteins as inhalant anesthetics can cause vasodilation and decreased venous return). Patients that are dehydrated should have their deficits corrected as much as possible before induction of anesthesia. Hypoproteinemic patients may benefit from colloid administration. If there is a need to prevent or control vomiting after the procedure, maropitant (Cerenia) or dolasetron (Anzemet) are highly effective choices.
Procedure
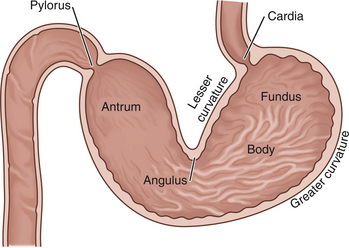
All areas of the stomach should be examined completely in every patient that undergoes gastroscopy. Therefore the beginning endoscopist must learn to identify landmarks properly (Figure 4-1). Only after the endoscopist has become familiar with luminal gastric anatomy do maneuvering the endoscope to obtain a retroflexed view of the cardia, advancing the scope around the incisura angularis to reach the antrum, and traversing the pyloric canal become consistent and effortless procedures.
Gastroesophageal Junction
Because the esophagus is essentially in a posterior plane compared with the stomach, the endoscope tip needs to be deflected in the distal esophagus before it can be successfully advanced to the stomach. As the endoscope is advanced to the distal esophagus, the position and configuration of the gastroesophageal junction are noted (see Chapter 3). The endoscope tip should be centered at the gastroesophageal orifice. As the scope is advanced, the tip is deflected to the left approximately 30 degrees with simultaneous slight upward deflection as the gastroesophageal junction is passed. In most patients this is easily accomplished by rotation of the outer control knob counterclockwise for left deflection and the inner control knob counterclockwise for upward deflection. In some patients, minimal or no upward deflection is needed. When the endoscope tip is properly directed, no resistance should be encountered as the scope is advanced to the stomach. If the tip is advanced too far before deflection is begun, the endoscope is usually directed into the esophageal wall bordering against the posterior aspect of the lesser curvature of the stomach. If this occurs, the endoscope tip should be retracted and repositioned. Variable degrees of air insufflation of the distal esophagus may be necessary to aid visualization and positioning. In general, air is continually insufflated as the endoscope is advanced along the esophagus and through the gastroesophageal junction (keep fingertip on the air insufflation button).
Proximal Stomach and Gastric Body
The endoscope tip should be positioned just through the gastroesophageal junction so that the endoscopist can become spatially oriented and obtain an overview of the gastric lumen. As the tip enters the stomach, the rugal folds, generally on the greater curvature of the body, are seen. Often the stomach walls are partially or completely collapsed, especially in medium to large dogs or if only a small volume of air was insufflated during esophagoscopy. In this situation the view of the stomach is quite limited, and it is necessary to first pause and insufflate air before attempting to advance the scope. Without sufficient gastric distension, the endoscopist will have difficulty identifying the key landmarks and performing a complete examination of the stomach.
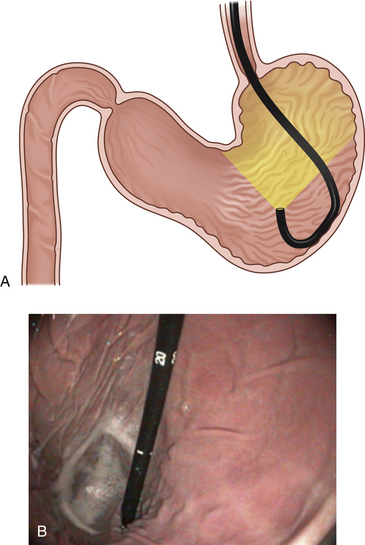
The ideal degree of gastric distension is a matter of judgment. Generally the distension should be at least to the point that the rugal folds begin to separate. This allows for spatial orientation and the identification of most gross abnormalities, such as an ulcer, a mass, or a foreign body. In cats and small dogs the degree of insufflation can be achieved within seconds; in giant breeds, constant insufflation may be necessary for 30 to 120 seconds before adequate distension is achieved. Later in the procedure it may be necessary to distend the stomach to a greater degree so that the entire gastric mucosal surface can be carefully examined (Figure 4-2).
On the other hand, during insufflation the endoscopist must be careful not to overdistend the stomach because this may result in significant cardiopulmonary compromise. When the stomach is overdistended, the rugal folds become almost completely flattened or undetectable, superficial blood vessels can sometimes be observed, and the mucosa may appear blanched (see Figure 4-2). The respiratory rate may increase significantly. The endoscopy assistant should be constantly aware of changes in the character of the patient’s respirations and any increase in anterior abdominal distension. As soon as possible a sufficient volume of air should be suctioned off to moderately deflate the stomach. During most gastric examinations, both air insufflation and suction are commonly used to maintain a proper and safe balance of distension.
As the scope is gradually advanced through the proximal stomach, the endoscopist can thoroughly evaluate the gastric body by using the control knobs to deflect the endoscope tip or by rotating the insertion tube with the right hand (torque). With the patient in left lateral recumbency and the endoscope held in a conventional manner (i.e., buttons up), the endoscopic view is predictable. The smooth lesser curvature is on the endoscopist’s right, and the rugal folds of the greater curvature are seen below and to the left (Figure 4-3). Required directional changes can usually be made with the left thumb on the inner control knob and the right hand controlling rotation (torque) of the insertion tube. In most cases, only minor directional changes are needed to provide a panoramic view. The endoscope is advanced along the greater curvature until the angulus is identified. The angulus appears as a large fold that extends from the lesser curvature. The angulus is an important landmark that separates the body of the stomach from the antrum. Once the angulus is identified, the lesser curvature can be easily differentiated from the greater curvature of the stomach. With the patient in left lateral recumbency, the antrum is directed up or away from the tabletop. As viewed from the gastric body, the angulus and entrance to the antrum usually appear as a circular or crescent-shaped orifice that is smaller than the distended body of the stomach (see Figures 4-18, D; 4-19, B; and 4-41). The endoscopist must be able to maneuver around the angulus to advance the endoscope to the antrum, pylorus, and duodenum.
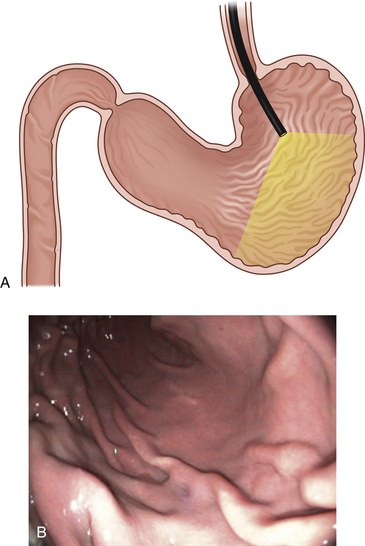
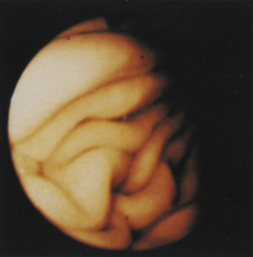
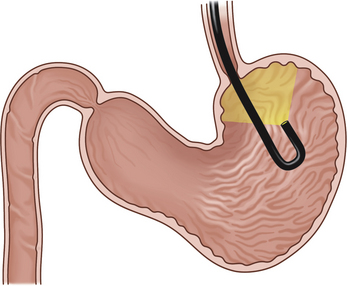
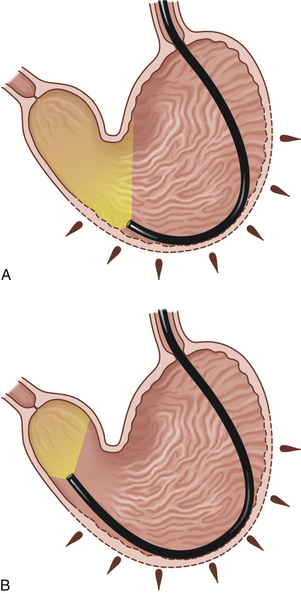
Figure 4-3 The initial view as the endoscope is advanced from the esophagus into the stomach is of the greater curvature. As described in the text and in Figure 4-2, the endoscopist first pauses to insufflate air to begin distending the stomach. If the endoscope is advanced straight ahead without adequate insufflation, the scope tip will engage the gastric wall and visualization will not be possible. A, Areas of the stomach not in view (shaded) as a standard forward-viewing endoscope is advanced into the proximal stomach. A retroversion maneuver is required to completely view the cardia and fundus. B, Corresponding endoscopic image with moderate air insufflation. The rugal folds and greater curvature of the stomach are clearly visualized, and this appearance is normal. The angulus fold is not in view.
Once the angulus and proximal antrum are visualized, the endoscopist has the option of advancing directly through the pyloric canal to the duodenum or completing the gastric examination. The cardia and fundus have not yet been completely evaluated. Depending on the particular endoscope’s angle of illumination, a portion of the proximal stomach cannot be seen as the scope enters the stomach (see Figure 4-3). For the cardia and fundus to be visualized the endoscope must be retroflexed (termed retroversion or J maneuver) so that it is possible to see the portion of the scope entering through the cardia, as well as the surrounding area (Figure 4-4). The retroversion maneuver should be done either at this point or after duodenoscopy. It can be advantageous to proceed directly from the angulus to the pylorus and duodenum. The physiologic function of the pylorus is to close in response to gastric distension. When gastric distension has been kept to a minimum and the antrum is not actively contracting, the pylorus is in a relatively lax state and the endoscope can pass through it with only minimal resistance. However, if a large volume of air has been insufflated and the endoscope has been significantly manipulated in the stomach, the pylorus may be tight and difficult to traverse. In my experience this is a greater problem in large breed dogs. Also, as the endoscopist becomes more experienced, it becomes easier to maneuver through difficult areas. I usually prefer to perform at least a cursory examination of the entire stomach before proceeding to the duodenum (i.e., the retroversion maneuver is performed before advancing to the pylorus). In most cases I perform a final, more thorough gastric examination and procure biopsy specimens after duodenoscopy.
Retroversion (J Maneuver)
To provide an en face view of the angulus, the endoscopist must initiate the retroversion maneuver at a point proximal to or opposite the angulus (see Figures 4-24, 4-25, and 4-26). The scope is advanced along the greater curvature to the level of the distal body. The inner control knob is turned counterclockwise with the left thumb, and as the endoscope is gradually advanced, the angulus can be seen en face. Variations of normal appearance may be present (see Figures 4-18 through 4-32). The endoscope tip is then deflected upward as far as possible (full counterclockwise rotation of the inner control knob) as the scope is advanced a little farther with the right hand. This maneuver generally requires at least 180 degrees of tip deflection. Most newer endoscopes are capable of 210 degrees of upward tip deflection. This deflection provides a retroflexed view of the endoscope as it enters the stomach through the cardia (see Figure 4-4). Pulling the endoscope back once this view is attained draws the endoscope tip closer to the cardia (Figure 4-5 and see Figure 4-25). A circumferential examination of the proximal stomach is completed by rotation of the insertion tube (torque) or by turning the outer control knob in each direction for lateral deflection. Air insufflation is usually necessary to keep the proximal stomach dilated. If there is insufficient distension, the stomach walls will collapse around the endoscope and it will not be in view. During most of the examination the right hand is kept on the insertion tube to keep the tube in place with respect to forward and backward motion.
In cats the retroversion maneuver is started when the endoscope tip is in the midbody area (see Figure 4-43). The tip is deflected upward as the endoscope is advanced. Because the working area is smaller than in most dogs, an en face view of the angulus similar to what is seen in dogs is not achieved as often in cats.
Paradoxic Motion and Inadvertent Retroversion
Once the endoscopist has mastered a few basic techniques, the body and antrum can be quickly traversed in most cats and small- and medium-sized dogs. Occasionally, however, in some medium- and large-sized dogs, advancing the endoscope to the antral canal and pylorus can be quite difficult. Many beginning endoscopists often feel “lost” as they try to maneuver a scope in the stomach of a large dog. The most bothersome occurrence is the formation of a loop in the insertion tube as it passes along the greater curvature. As the tip of the endoscope is advanced toward the antrum and pylorus, the insertion tube invariably comes to lie along the greater curvature. The stomach can stretch considerably to accommodate intraluminal forces, and much of the forward force generated by the advancement of the scope is absorbed by the greater curvature so that the curvature is pushed caudally in the abdomen. A loop may form against the greater curvature (Figure 4-6). Endoscopically it may appear that as the insertion tube is advanced farther, the instrument tip is not moving in response or is actually moving away from the pylorus; this is termed paradoxic motion. Loop formation can occur readily, especially with newer, more flexible endoscopes and longer narrow-diameter instruments, as well as when the stomach contains a large volume of air. To reach the pylorus, the endoscopist should continue to advance the endoscope until the greater curvature loop is fully formed and the tip begins to move forward again (see Figure 4-6, B). Further upward deflection of the tip using the left thumb on the inner control knob may be necessary. There will be mild resistance in some cases as the endoscope is advanced along the greater curvature, and the endoscopist should continue to push through this area while making tip direction changes, as needed, to keep the scope tip in line for passage into the antral canal.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree