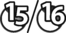7 Fungal diseases
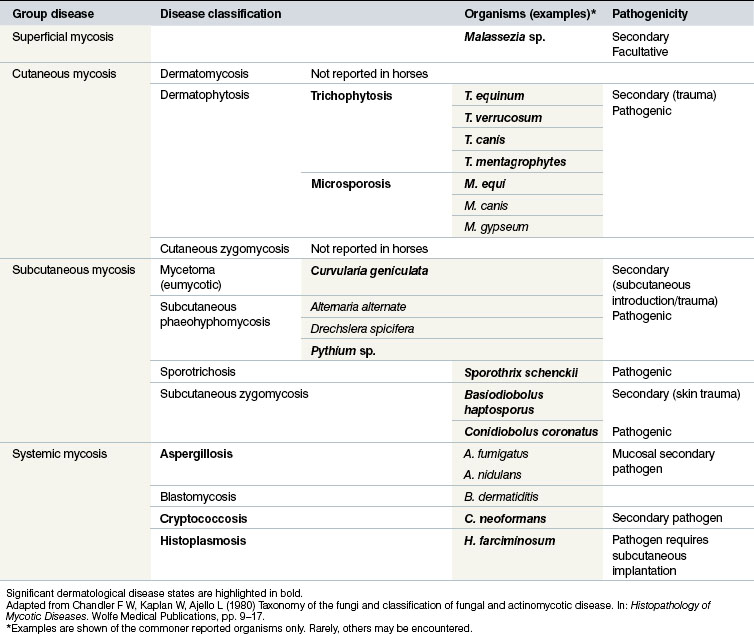
Classification of the fungal dermatological infections in other species is usually based on the location of the infection. This system is also applicable to equine mycotic disease (Table 7.1). Three broad groups of fungal skin disease can be identified:
1. Superficial mycoses are those in which the pathogen is confined to the stratum corneum (and does not affect hairs). As might be expected, there is little or no tissue reaction or inflammation and as result they tend to be rather benign. Some of the conditions are viewed as facultative pathogens, or even commensals, rather than primary pathogens and in either case there can be secondary seborrhoeic changes that result in excessive scale and flake on the skin surface. There is usually little immunological or cellular response associated with the organisms and no obvious seroconversion. A good example of this is Malassezia spp. infection, which has recently been recognized as a potentially significant skin infection in horses.
2. Cutaneous mycoses affect all keratinized tissue including hair, horn and skin, although most of the pathogens are confined to the non-living layers of the skin and the appendages. The organisms are capable of causing significant destruction of keratinized tissue and there are usually immunological responses in the host that result in obvious lesions and seroconversion with variable protective properties.
3. Subcutaneous/deep mycoses constitute a heterogeneous group of fungal diseases that are more deep-seated. They involve subcutaneous tissues, possibly in addition to the dermal/epidermal involvement. Whilst most of these remain localized (e.g. Alternaria alternate), some do spread insidiously to contiguous tissues, e.g. Basidiobolus haptosporus and Conidiobolus coronatus infections. Some spread via lymphatic vessels occurs, e.g. histoplasmosis (epizootic lymphangitis) due to H. farciminosum and sporotrichosis due to Sporothrix schenckii. There may be difficulties with diagnosis and treatment in this group of diseases. Seroconversion may take place but seldom seems to result in an effective protective process. Most of the conditions are chronic and progressive.
There are also some incidental and secondary fungal infections. Therefore, a definitive diagnosis is often dependent on the clear and unequivocal detection of a known pathogenic organism.
Trichophytosis
Profile
The most common species are Trichophyton equinum var. equinum (TEvE), T. equinum var. autotrophicum (TEvA), and less commonly T. verrucosum (most often from direct or indirect contact with infected cattle) and T. mentagrophytes (most often derived from contact with infected rodents and cats) (Pascoe 1979, 1984). T. equinum var. equinum tends to be the predominate species in the northern hemisphere while T. equinum var. autotrophicum tends to dominate in the southern hemisphere and the Antipodes.
Infection relies upon the presence of active (live) spores and mechanical skin abrasion (even if very mild) and this is the reason for most lesions developing on girths and saddle and jockey boot friction areas. The spores become vegetative in the damaged stratum corneum and the fungal hyphae penetrate the anagen hair follicles. In Trichophyton species infection, the hyphae invade the hair shafts and relatively few spores are produced within the hair shafts (endothrix spores). As the fungal hyphae penetrate downwards towards the hair bulb, keratolytic enzymes are produced. These enhance and facilitate further penetration. Damage to the anagen hair shaft occurs so that the outer portion is shed; the fungus may be expelled with it. In order for the fungus to thrive the hair has to be actively growing and so as soon as the hairs enter the telogen stage the fungus cannot easily survive. At this stage the highly resistant spores are produced and may be shed into the environment with the hair shaft. Most cases resolve spontaneously as a result of hair shedding in telogen phase or as a result of hair breakage or following an immuno-excitatory inflammatory folliculitis; this can be a result of local hypersensitivity responses to the secretions of the fungus. In some cases the complex of secretory and induced inflammatory products results in a more florid inflammation – often with some mild irritation to the horse. Following infection, lesions are visible at around 7–21 days depending largely on the immune status of the horse. Reinfection of a single hair follicle and its hair shaft does not occur until the hair re-enters its anagen phase. If immunity is strong at this stage, the infection is unlikely to re-establish. Clinically the lesions may expand and continue to spread across the horse for some 2–4 months depending on the stage of hair growth and the extent of immunity. Immunity to Trichophyon spp. fungi is short-lived; there are some common antigens and so reinfestation with another Trichophyton species is less likely. However, there does not appear to be any significant correlation between circulating antibody concentrations and the extent of resistance to reinfection. The natural antifungal effects of healthy untraumatized skin seem to be at least as important in the overall resistance to the disease. Repeated degreasing shampoos are probably not helpful because sebum has a significant protective property.
1. Very common cutaneous mycosis due to Trichophyton spp. dermatophytes. Worldwide distribution. Spores are highly resistant so repeated infections occur in stables/yards. The fungus requires epidermal damage to gain entry and remains inside the hair follicle and on the hair shafts. The sites of infection reflect areas of superficial skin trauma such as tack and harness contact points. Generalized infection can follow simply from grooming with infected brushes or transferring the fungus to fresh sites on the same horse. The spores can survive for many years.
2. Early clinical signs are erect hairs, some local swelling/ oedema with mild exudate in a few cases. As all hairs are involved within a local area, complete shedding of hair occurs and lesions are easily and completely epilated leaving a silvery exposed epidermis. Abrasions from jockeys’ boots and girths are common sites for infection.
3. Diagnosis is relatively simple clinically but differential culture is important for control measures. Biopsy can be helpful.
4. Treatment involves isolation of affected horses and careful hygiene to prevent spread between horses and to humans. Topical antifungal washes are effective and oral griseofulvin can be given; it should not be given to pregnant mares. Envi-ronmental control is also important to limit the risks of spread so fungicidal disinfectants are used as sprays and washes for tack and harness, etc.
5. Control by vaccination is possible in some countries but early recognition and isolation of cases, and stable and personal hygiene are by far the best ways to prevent its spread. Individualized tack, harness and rugs, etc. are essential, especially in stables with a history of dermatophyte infection. When handling any case of dermatophytosis, gloves should always be worn.
Clinical signs
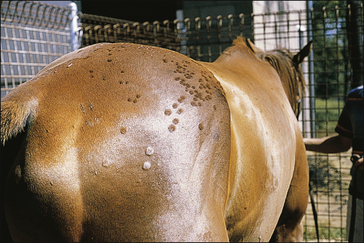
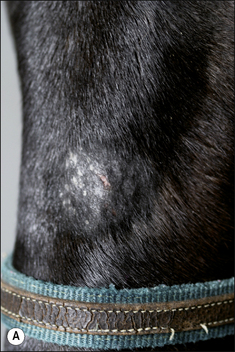
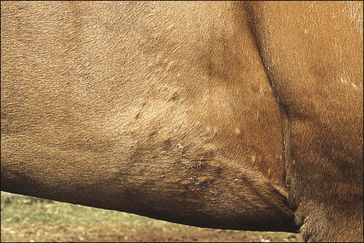
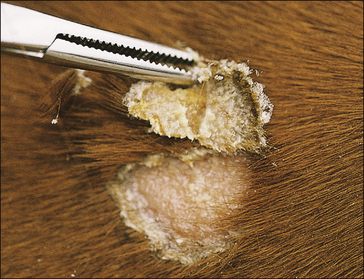
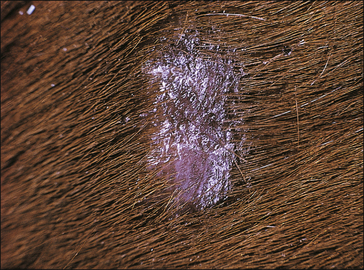
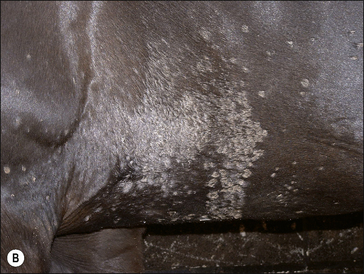
The earliest lesions appear as erect hairs in circular areas of 5–20 mm diameter (Fig. 7.1). There is often a degree of localized inflammation resulting in a thickening of the skin within the infected area; this can be urticarial in nature and there may be some exudate, which dampens the site. By day 7–10 post-infection hair can easily be plucked from the site (Fig. 7.2), leaving a silvery, slightly reddened circular area of exposed epidermis (Fig. 7.3). Hair loss also occurs naturally but this is less abrupt and so the lesions may not be obvious until 14–21 days. One of the cardinal signs is the ease with which the hair is removed – this is because most (probably all) hairs are affected within the lesion (Fig. 7.4).
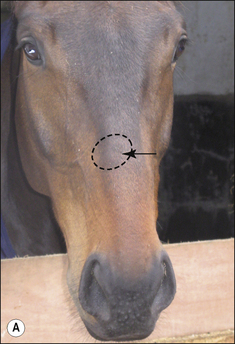
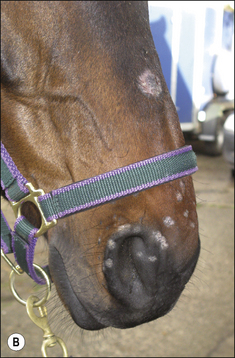
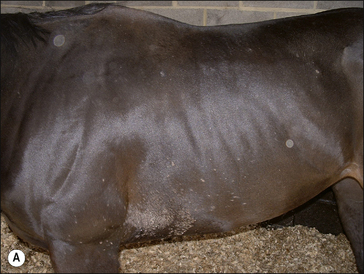
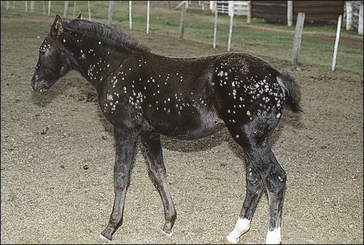
Girth and shoulder/chest wall areas are common sites owing to infection from contaminated girths, riding boots, etc. (Fig. 7.5). Generalized infections are also common, particularly in younger horses (Fig. 7.6).
Different species of Trichophyton such as T. verrucosum and T. mentagrophytes, which are respectively usually derived from infected cattle and rodents, show some differences in the type of lesion (Fig. 7.7 and Fig. CD7 • 1A–D)  .
.
Differential diagnosis
• Microsporum infection: this is a similar condition epidemiologically and clinically but tends to involve only a proportion of the hairs in an infected area; plucking of the hair is therefore more difficult and is sometimes resented.
• Dermatophilosis (Dermatophilus congolensis): characteristic bacteria and epidemiology; tends to infect the back and lower limb legions mostly and lesions are more purulent.
• Culicoides allergy/hypersensitivity (sweet itch): severe localized pruritus mainly centred on the mane, tail and in some cases the ventral abdomen, associated with seasonal exposure to Culicoides spp. in particular.
• Insect bites: defined localized swellings associated with oedema and a central haemorrhagic spot.
• Mite or louse infestation: obvious parasites and more pruritus; localized to limbs and head and tail; no defined lesions usually present.
• Pemphigus foliaceus: this can be very similar clinically and even histologically and it may require special stains to identify the fungal elements.
• Sarcoidosis: generalized exfoliative seborrhoeic disease with little similarity; some generalized forms of trichophytosis can be similar but they tend to self-cure and mycology and skin scrapings are diagnostic.
• Granulomatous enteritis syndrome: systemic involvement with prominent scaling.
• Alopecia areata: similar circular areas but with characteristic absence of any inflammatory responses and sterile cultures; characteristic histology.
• Anhidrosis: single cases affected with typical history of a move to a warm climate; affected cases may develop secondary trichophytosis due to impaired local immunity.
• Actinic dermatitis: restricted to white regions, particularly on the nose, face and distal limb regions.
• Mercurial poisoning: history of applications.
• Wound/exudate/lacrimal scalding: history and obvious clinical evidence; may be secondarily affected with trichophytosis due to skin damage.
Diagnostic confirmation
• Characteristic clinical signs and history of contact with infected horses (or other species).
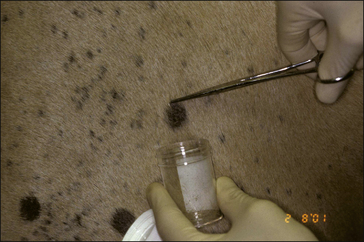
• Hair plucking from the margins of fresh lesions (Fig. 7.8) can be examined microscopically (possibly after clearing with chlorolactophenol or 10% potassium hydroxide solution). Hyphae and relatively few large endothrix spores will be seen.
• Staining with lactophenol cotton blue can assist the recognition of the hyphae.
• Infected hairs do not fluoresce under ultraviolet light.
• Culture of hairs plucked from the margins of lesions on Sabouraud’s fungal medium. Medium with added phenol red provides an early indicator of dermatophytosis. Culture permits easy identification of the typical macroconidia.
• Skin biopsy is usually diagnostic of dermatophytosis but does not help to establish the species concerned (upon suspicion the pathologists should be informed so that special stains can be used to confirm the hyphae and spores).
Treatment
Most cases will resolve spontaneously after 6–12 weeks (particularly if the horses are in sunshine) (Pascoe 1973a). Treatments do not shorten the course but may limit the spread of infection and limit the extent of environmental contamination. Subsequent immunity can last for an extended period but some cases can re-emerge following partial elimination from hair follicles.
Oral griseofulvin may be administered daily for 15–60 days (Hiddleston 1970) but the results of this alone are very variable. There are no reports of its efficacy and many specialists consider that it is of no material help. In any case, it probably does not reduce the infectivity of the spores and fungus-laden hairs. It should therefore not be used alone except perhaps in grazing horses. The drug is teratogenic and must not be used in pregnant mares.
Control
The stable environment can be effectively disinfected by ‘fogging’ with potassium monopersulphate (using an industrial or horticultural fogging machine) or enilconazole distributed in the same fashion (Desplenter 1989).
Contaminated tack and other equipment may be washed in suitable fungicidal disinfectants; modern halogenated peroxygen compounds have a strong sporicidal and antifungal effect, but these can be unreliable. Preferably, all tack and equipment should be fumigated with formaldehyde gas (see p. 86).
Microsporosis
Profile
Ringworm due to Microsporum gypseum, M. equinum or M. canis (microsporosis) is less common than trichophytosis (see above). This disease is also highly contagious, being spread by direct and indirect contact with infected horses or through contaminated equipment or environment. It can also be spread by biting insects and skin abrasion (Pascoe & Connole 1974).
1. Microsporum spp. are less common in most circumstances than Trichophyton spp. infections. Isolated lesions are more common than extensive coalescing areas. The pathogenesis is probably the same as trichophytosis. The common species include M. equinum, M. canis and M. gypseum. The origin of the infection is an important aspect of the epidemiology and control. The spores and the active mycelia can survive for months in bedding, wooden poles and tack/blankets and harness.
2. Clinical signs show first as small expanding areas of localized oedema resembling urticaria (some cases are complicated by urticarial reactions). The lesions may have significant exu-date in the early stages and may be mildly pruritic – the horse may rub the affected site against a solid object but does not bite at the lesions. Plucking of the hairs is resented because not all the hairs are equally affected and so many are still firmly attached. This makes the lesions far less distinctive.
3. Diagnosis is made on the clinical signs and the fungal cul-tures and microscopy of stained smears and cleared hairs. Differentiation from urticaria is relatively simple but secondary urticaria-like plaques can arise as a result of microsporosis. Other similar cutaneous lesions occur in some forms of pem-phigus foliaceus, sarcoid and insect bite reactions.
4. Treatment involves local topical washes with fungicidal compounds such as natamycin, enilconazole, or miconazole. The whole body should be washed thoroughly and then the hair clipped off the defined lesions and these areas washed again. Environmental disinfection is important. Sunshine is a strong inhibitor of Microsporum spp. fungi.
5. The prognosis is excellent – most cases will resolve spon-taneously within 6–12 weeks but the risk of spread to other horses and to humans means that treatment should be instituted.
The pathogenesis is probably indistinguishable from trichophytosis (see p. 168).
Clinical signs
Small alopecic areas, most commonly on the face and legs, develop but lesions may also follow the distribution of insect bites elsewhere (Fig. 7.9). The lesions may be mildly exudative and many have an oedematous (urticaria-like) plaque within the affected skin.
Not all the hairs in a particular area will be equally affected and so, when a lesion is plucked, not all the hairs are shed. Plucking of the lesion is therefore more difficult and is often resented by the horse (Fig. 7.10).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Key points: Trichophytosis
Key points: Trichophytosis










 Key points: Microsporosis
Key points: Microsporosis