3 Diagnostic/investigative procedures
The equipment required to collect specimens for diagnostic purposes in shown in Table 3.1. This list is really very basic and there is no real j42ustification for collection of inadequate samples. The return from the pathologists is proportional to the care with which samples are collected and handled.
Table 3.1 Equipment that can be used to collect dermatological samples from the skin
A basic practice laboratory should have the equipment listed in Table 3.2. Sophisticated or expensive equipment is not usually necessary in practice, and for procedures that require the more advanced expertise of a pathologist the specimen should be sent directly to that specialist. Without the experience or skill required to perform sophisticated tests, possession of sophisticated apparatus is often wasteful and frustrating. There is little merit in sending prepared samples to a pathologist (such as mounted and stained sections) unless a second opinion is being sought – indeed the pathologist may not then be able to establish the true orientation of the specimen and might be unable to explore other stains and other orientations of the sample. When a pathologist receives a fixed specimen with a full history and a good clinical description of the clinical case (possibly even with photographic images of the sampled lesions) he/she will usually cut the specimen into the most appropriate ‘blocks’ for processing and in that way the best possible diagnostic information can be gleaned. Many of the problems encountered in diagnosis are the result of inadequate information, inappropriate specimen selection and inappropriate interpretation of the results of poorly performed analysis.
Table 3.2 Basic equipment for a simple practice laboratory
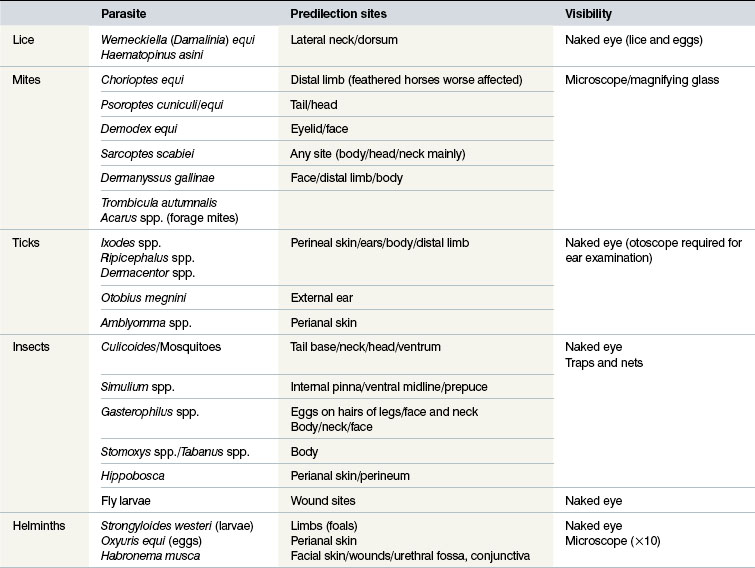
ARTHROPODS THAT CAUSE EQUINE DERMATOSES
Mites
Sarcoptes scabiei
Identification
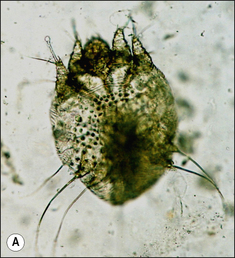
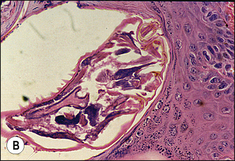
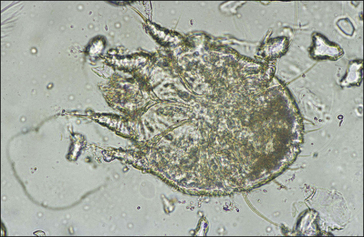
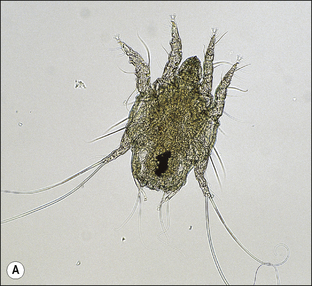
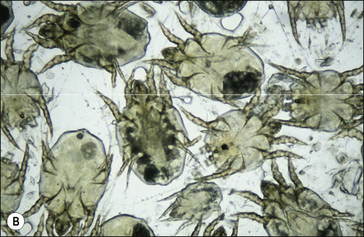
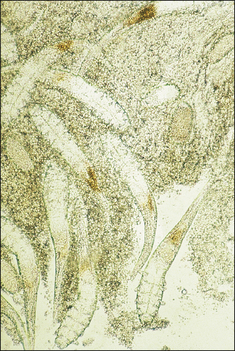
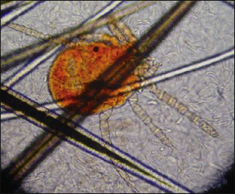
S. scabiei are round and flattened (Fig. 3.1A) with a rounded head region and short front legs. The first pairs of legs have tiny claws and end with a stalk (the pretarsus) bearing a sucker. The two pairs of posterior legs, which bear long hairs (setae), do not extend beyond the body margin; the dorsal surface bears cuticular folds giving rise to a ‘thumb print’ pattern and is covered with scales and strong spines recognizable in histological sections (Fig. 3.1B).
Life cycle
The entire life cycle takes place on the host. Following mating the female mite tunnels into the sub-corneal layer of the skin. Each tunnel (1.0–2.0 cm in length and just below the skin) contains a single female mite, her eggs and excreted material. The female mite lays around three eggs per day and eggs hatch to six-legged larvae within the tunnels after 3–4 days. The larvae exit the tunnels and successive moulting to eight-legged protonymph, tritonymph and female (600 μm) and male adult (300 μm) stages occurs. Newly moulted adult females mate immediately and create new tunnels by eating skin and tissue fluids. The total egg-to-adult life cycle can be as little as 11 days but up to 3 weeks is usual.
Psoroptes equi
Identification
The body of P. equi is oval-shaped and the head region is pointed (Fig. 3.2). All stages of this mite are identified by the presence, on the first two pairs of legs, of a three-segmented stalk which ends with a trumpet-shaped sucker. The males are identified by the presence of copulatory suckers in addition to the above features, which are specific to the genus Psoroptes. These characters enable differential identification from Chorioptes equi, a more common mite of equines, and from incidental forage mites, some of which have a broadly similar morphology.
Significance
Psoroptes equi infestations in the horse occur in regions of thick hair such as the tail and mane but also on the face, ears and other parts. A fluid exudate, which dries leaving crusts, is produced. Thickening and hair loss can occur and is particularly noticeable along the margins of the pinnae. In severe infestations large numbers of active mites can be recovered from the edge of lesions. Psoroptic mange may result in emaciation, head shaking and pruritus (see p. 193).
Chorioptes equi
Identification
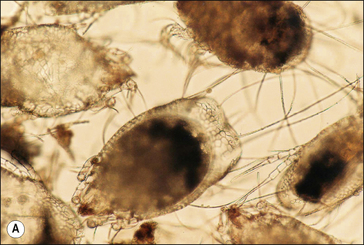
Females (Fig. 3.3) (300–400 μm) and males (300 μm) are significantly smaller than P. equi and have a rounded head region. Chorioptes do not have the jointed stalk of Psoroptes and the ending is cup-shaped as opposed to trumpet-shaped. There are long whip-like setae on the reduced third pair of legs in the female. In the male, the two broad posterior lobes are square-shaped and bear numerous long hairs. In heavy infestations all stages of the life cycle including eggs are readily encountered and adult C. equi are often seen as mating pairs.
Demodex spp. (follicle mites)
Clinical disease due to Demodex spp. is extremely rare in horses and the parasite is usually regarded as a commensal and facultative pathogen. Two species of host-specific Demodex affect horses; D. equi is found on the body and the larger species, D. caballi, infests the eyelids and muzzle.
Identification
Demodex spp. mites are minute (100 μm) with a tapered and pointed abdomen, the cuticle of which is striated. The stumpy legs, which end in claws, and the cigar-shaped bodies are recognizable in stained skin sections (Fig. 3.4).
Significance
Although Demodex spp. are usually regarded as non-pathogenic, significant clinical disease can result if the health and immune status of the animal is compromised. Most cases are reported to be in horses receiving long-term glucocorticoid therapy. Infestation may present as scaling and alopecia; papules and pustules may form on the face, shoulders, neck and limbs (see p. 196).
Trombicula spp. (harvest bugs, berry bugs, chiggers)
Identification
Trombiculids (Fig. 3.5) are very small (200 μm) and are sometimes noticed in groups as pale orange-brown specks on the skin. Close examination will reveal six-legged larvae having a scutum and body covered with very small feathered hairs.
Life cycle
The adults and nymphs are non-parasitic and predate upon the eggs and larvae of various types of soft-bodied insects. Eggs are laid in well-drained soil and newly emerged larvae quest, in a similar manner to ticks, on the tops of grass stems awaiting a passing host. The mouthparts pierce the skin, producing an exudate on which they feed for several days. In northern Europe, there is one generation of larvae each year which are very active in late- to mid-autumn. Horses are parasitized through contact with grasses when feeding and during exercise.
Forage mites
Identification
These soft-bodied mites are oval-shaped and typically possess numerous and very long whip-like hairs on the posterior body margin (Fig. 3.6). Teeth are usually prominent on the palps. These features are important in distinguishing forage mites from the mites that cause mange.
Significance
When in contact with horse skin, mites may cause a hypersensitivity-related dermatitis or even abrade the skin to cause a temporary erythema. There may also be pruritus, papules and crusts. The most obvious lesions occur close to the hay or straw that harbour the mites, i.e. the face and distal limbs (see p. 198).
Dermanyssus gallinae (poultry red mite)
Identification
Adults are relatively large – up to 1 mm in length. The body cuticle is tougher than that of the soft-bodied permanent species such as Psoroptes spp. and the legs are spider-like legs that protrude well beyond the body margin (Fig. 3.7). A diagnostic feature that separates this mite from the similar types (below) is the anal shield, which is large and D-shaped (Fig. CD3 • 1) ; this feature is only seen if the specimen is cleared (see section examination for mange mites of horses). Poultry mites are fairly easy to see with the naked eye or with a magnifying glass, appearing bright red when fully blood-engorged and almost black when the blood meal is more than 24 hours old. Pigeons’ nests within or near stables may be a transient source of D. gallinae in autumn when young birds are fledged.
; this feature is only seen if the specimen is cleared (see section examination for mange mites of horses). Poultry mites are fairly easy to see with the naked eye or with a magnifying glass, appearing bright red when fully blood-engorged and almost black when the blood meal is more than 24 hours old. Pigeons’ nests within or near stables may be a transient source of D. gallinae in autumn when young birds are fledged.
Life cycle
Mites are fully fed within a few hours of arriving on their host. They then drop off quickly. Eggs are laid near nests and roosts. Newly hatched larvae do not feed but undergo successive moults to the nymph and adult stages in as little as 7–10 days. Populations can build up rapidly in bird accommodation.
Examination for mange mites of horses
Where infestation with non-burrowing mites (e.g. Psoroptes and Chorioptes) is suspected, repeat surface samples should be obtained using a strong comb or preferably a stiff brush (see p. 60). Deeper skin scrapings using a scalpel blade are necessary to recover burrowing mites such as Sarcoptes and Demodex.
Ticks
Hard ticks (family Ixodidae) and soft ticks (family Argasidae) are blood-feeding ectoparasites of animals and birds found throughout the world. Hard and soft ticks of animals are classified in nine major genera found in temperate, topical and subtropical parts of the world (see p. 212). These two groups exhibit different feeding behaviour, life cycles and morphologies. Ticks cause serious mechanical damage, irritation, and inflammation to the skin. Infestations can also induce hypersensitivity.
All stages of ticks (both hard and soft) possess a characteristic hypostome, a toothed structure which anchors the tick into the skin. No other arthropod groups, e.g. mites, possess this feature. Feeding hard ticks tend to aggregate and remain attached to the host for several days, usually provoking a significant skin reaction. Deeper lesions are caused by species in the hard tick genera Amblyomma (Africa, North and South America, Asia) and Hyalomma (Asia and Africa) because they possess very long mouthparts.
Life cycles
Hard and soft ticks comprise the parasitic stages of larva, nymph and adult. Soft ticks have a multi-host developmental cycle. Each stage of the soft tick life cycle feeds several times and for only a few minutes on each occasion. Hard ticks are far more important and their life cycle is now described in detail. Adult hard ticks spend the first 24 hours on the host without imbibing blood and at this point females are more or less flat and 5–10 mm in size. Females attach to the host, attract males and become fully engorged with blood after 6–12 days. Engorged adult ticks may reach 1 cm in size (Fig. 3.8); they then drop off the host and produce thousands of eggs in vegetation.
Some species of Hyalomma and Rhipicephalus spp. are two-host ticks.
Significance
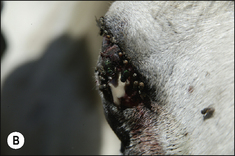
Hard ticks tend to feed at specific sites, especially the ears, face, neck, axillae, groin, distal limbs and tail, but although the different species have preferential feeding sites, ticks may be found anywhere on the body. Due to the time spent on the host, one-host and two-host species are likely to induce a more severe host reaction than three-host species. The cutaneous signs associated with hard tick feeding in horses include focal necrosis and a variable (mild–severe) inflammatory response. Papules, pustules and ulcerations occur. The larvae of Boophilus microplus are reported to cause a local hypersensitivity reaction with papule formation at the site of feeding. Tick feeding can cause abscesses by the introduction of surface bacteria. There may be subsequent infection with Staphylococcus. The lesions created by ticks may be attractive to screw-worm (fly) larvae in the tropics and subtropics (see p. 212).
The larvae and nymphs of the soft tick Otobius megnini can produce a severe otitis externa.
Flies
All species of Diptera have a complex life cycle with a complete metamorphosis: larvae or ‘grubs’ hatch from eggs which are laid on various types of organic substrate and increase in size by feeding and moulting through three instars. Feeding flies can cause severe irritation, dermatitis and hypersensitivity. (Eosinophilic) collagenolytic granuloma within the deep dermis may be associated with blood-sucking flies.
Flies are a persistent problem for many reasons. Some simply cause worry, others transmit serious diseases and others cause direct pathology by either tissue destruction or debilitating parasitism (Figs 3.9 and 3.10).
Biting flies
Family Muscidae – Stomoxys calcitrans, the ‘stable fly’ or ‘biting house fly’; S. niger; S. sitiens
Stomoxys calcitrans is the most important species found worldwide. S. niger and S. sitiens are common species in Africa and east Asia, respectively. Stable flies are known to transmit equine infectious anaemia and ‘surra’ (a form of trypanosomiasis). They (and the non-biting Musca spp.) are vectors of the stomach worm Habronema spp. and the eye worm Thelazia spp. in horses, the larvae of which cause cutaneous lesions. (see p. 217).
Identification
Stomoxys spp. are 5–7 mm, brown/grey and house-fly-like in appearance (Fig. 3.11). The abdomen is chequered and the thorax striped. Males and females are similar and possess a typical strong proboscis which is black, shiny and forward pointing. The palps are short and thin and very small compared to the length of the proboscis.
Life cycle
Stomoxys spp. breed in sheltered animal manure mixed with straw and urine but other varieties of rotting matter provide suitable breeding substrates, provided it is protected from rain. Larvae feed and moult from the first instar to the second and then to the third instar. Larval development may take as little as 7 days. The third-stage larva pupate and adult development within this is temperature-dependent. Stomoxys spp. overwinter as puparia in temperate regions but breed all year round in warm climates. In northern Europe, population levels peak in August/September and flies can be seen pestering housed and grazing horses. They can be recognized at rest ‘sunning’ themselves on exposed walls, farm machinery and beams.
Lyperosia irritans (=Haematobia irritans), ‘hornflies’; L. exigua
Identification
Lyperosia spp. are the smallest of the biting muscids. The palps are the same length as the proboscis. The head is rather small compared to the overall body of the fly and the wings are elongated (Fig. 3.12). Flies may appear embedded in the hair along the backline.
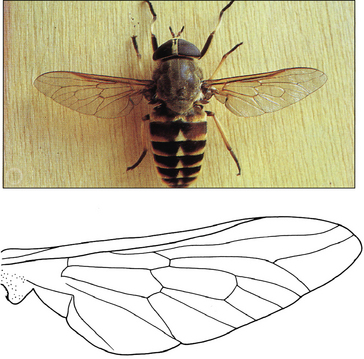
Family Tabanidae: Tabanus (=Hybomitra) spp. (horse flies, thunder flies); Haematopota spp. (cleggs); Chrysops spp. (‘green heads’)
Identification
Flies in the family Tabanidae are all large and stout-bodied, between 10 and 15 mm in size. Tabanus spp. are the largest of the Tabanidae, and measure 1–2 cm (Fig. 3.13). Like all tabanids the eyes are semi-moon-shaped and iridescent, the antennae are three-jointed and the wings are clear. Haematopota spp. are smaller (Fig. CD3 • 3A) and the wings are mottled. In Chrysops spp. (Fig. CD3 • 3B)
and the wings are mottled. In Chrysops spp. (Fig. CD3 • 3B) , the wings are usually banded.
, the wings are usually banded.
Life cycle
The larvae are carnivorous and develop from eggs laid underneath hanging vegetation in or near wet areas, muddy patches or marshes. There is usually only one generation of adult flies annually in temperate climates; emergence starts on warm days in early summer and feeding activity, on warm, humid days, is greatest in July and early August. Tabanids overwinter as late-stage larvae in Europe. Adult emergences overlap in tropical and subtropical zones, but are influenced by wet and dry times of the year. Females of some tabanid species do not require an initial blood meal to produce eggs, a phenomenon known as autogeny.
Family Hippoboscidae: ‘keds’ or ‘forest flies’; Hippobosca equina, H. rufipes, H. maculata
Identification
Adult flies are approximately 10 mm in length and are pale reddish brown (Fig. 3.14). They have small heads with bulging eyes and the proboscis is forward-pointing. Hippoboscids feed on the host for long periods (see p. 210).
Significance
The preferred feeding sites are the perineum and between the hind legs. Flies may be well tolerated by donkeys and horses that are exposed to high, persistent challenge throughout the fly season. This species can be a source of intense irritation in other circumstances, e.g. when horses are moved into infested areas (see p. 210).
Family Culicidae (mosquitoes): Anopheles spp., Aedes spp., Culex spp., Culiseta spp.
Life cycle
Only females feed on blood. Eggs are laid in batches on still surface water. The larvae and puparia of anophelines require unpolluted fresh water sites whereas culicines breed in stagnant, organic-rich water. The eggs of Aedes spp. have adapted to survive desiccation and are laid on moist substrates and not directly on water itself.
Family Ceratopogonidae: Culicoides spp. (midges); family Psychodidae: Plebotomus spp., Lutzomyia spp. (sandflies)
Significance
The very common skin disorder insect bite hypersensitivity (‘sweet itch’ Queensland itch) is frequently caused by hypersensitivity responses to the bites of Culicoides (see p. 284). This is a predictable highly pruritic condition in susceptible horses with self-inflicted skin damage through rubbing on posts, etc. The withers, mane, tail, face, ventral midline and other regions are often affected, leading to hair loss, excoriation and crusting which leads to hyperkeratosis and thickening of the skin.
Sandflies may also cause significant dermatitis in horses where this fly is endemic but this is usually transient (although some hair loss may persist) and usually far less severe than insect bite hypersensitivity (see p. 209).
Surface-feeding flies (‘nuisance flies’ or ‘sweat flies’)
Musca autumnalis (the face fly) and other Musca spp.
Hydrotaea irritans,‘the sheep head fly’, and other Hydrotaea spp.
Myiasis flies
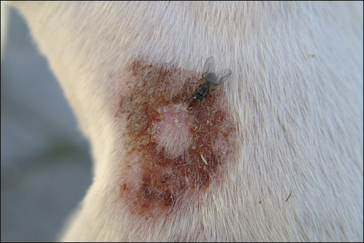
Myiasis is the invasion of living tissue by fly larvae and is a serious problem in livestock throughout the world. Fly larvae complete three instars while feeding and moulting in the hosts’ tissues. When wounds are involved, myiasis is said to be ‘traumatic’; where larvae mature inside a boil, the form of myiasis is termed ‘furuncular’ (see p. 207).
Facultative species: Lucilia spp.; L. cuprina (‘greenbottles’)
Identification
Lucilia sericata are metallic green flies (Fig. 3.15 and Fig. CD3 • 9A ) and measure approximately 10.0 mm. Their eggs are creamy white, approximately 1 mm in size and may be seen in groups or scattered on the skin. Second- and third-stage larvae a are 5–10 mm in size and are segmented and barrel-shaped. The puparia are tan-brown.
) and measure approximately 10.0 mm. Their eggs are creamy white, approximately 1 mm in size and may be seen in groups or scattered on the skin. Second- and third-stage larvae a are 5–10 mm in size and are segmented and barrel-shaped. The puparia are tan-brown.
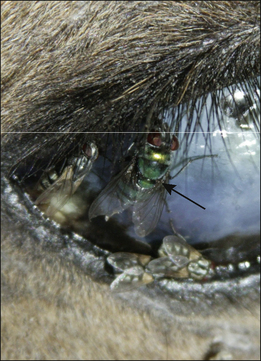
Figure 3.15 Lucilia sericata (arrow) feeding on a recently dead carcass. Note other species of fly are also present.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





 .
.


 . They possess a forward-pointing proboscis and hold their wings over the dorsal abdomen in a scissor-blade posture. The wings also have a characteristic hatchet cell not seen in other blood-sucking or nuisance fly species.
. They possess a forward-pointing proboscis and hold their wings over the dorsal abdomen in a scissor-blade posture. The wings also have a characteristic hatchet cell not seen in other blood-sucking or nuisance fly species.
 . Sandflies are small flies with hairy wings which are held erect above the thorax while feeding.
. Sandflies are small flies with hairy wings which are held erect above the thorax while feeding. . Musca sorbens, ‘the bazaar fly’ (Africa, Pacific Islands and Oriental regions, Australia), and Musca vetustissima, the Australian bushfly (Australia), are other important species.
. Musca sorbens, ‘the bazaar fly’ (Africa, Pacific Islands and Oriental regions, Australia), and Musca vetustissima, the Australian bushfly (Australia), are other important species. . Males are slightly larger and black in colour. H. albipunta resemble H. irritans but are 3–4 mm in size and the abdomen has a bluish hue.
. Males are slightly larger and black in colour. H. albipunta resemble H. irritans but are 3–4 mm in size and the abdomen has a bluish hue. ; Drymeia spp. closely resemble H. irritans but are darker (see Fig. CD3 • 8B)
; Drymeia spp. closely resemble H. irritans but are darker (see Fig. CD3 • 8B) . Fannia spp. are usually small, dark brown species with a pointed abdomen (Fig. CD3 • 8C)
. Fannia spp. are usually small, dark brown species with a pointed abdomen (Fig. CD3 • 8C) .
.