Fig. 38.1
A flow chart showing a series of conventional preparation procedures (a), freezing (b), in vivo cryotechnique (c), and cryobiopsy (d), followed by freeze-substitution (e) and immunostaining (f) or immunoelectron microscopy (g) for light or electron microscopic observation
In the case of the common quick-freezing of fresh resected tissues (Fig. 38.1b), it is another inevitable problem that only the organization of outer tissue layers is captured under well-freezing conditions. This is because ice crystal s of the animal organ tissue are easily formed at the time of a low-speed freezing. Therefore, with the HE-stained tissues of paraffin sections, we should carefully distinguish the deep tissue parts which are poorly kept at a slow speed of freezing condition. In such tissue areas, ice crystal sizes are gradually increasing from good areas of the directly contact tissue layer under well-freezing conditions to badly frozen tissue areas. The nucleoplasm of actively replicated cells with a little chromatin condensation in particular appears to easily produce lots of ice crystals in comparison with their cytoplasm. Moreover, it is believed that the water contents in cells and tissues at well-frozen sites are stored to be physically fixed as tiny amorphous ice , then keeping the intermolecular gaps. This usually facilitates the penetration of antibodies or probes at the time of immunostaining or labeling (Fig. 38.2b). When an organic acetone solvent is used as freeze-substitution liquid, we should consider another factor which improves the antibody or probe penetration because of lipid extraction of intracellular membranous systems (Fig. 38.2), including cell membranes or nuclear double membranes. On the other hand, quick extraction and movement of soluble protein s always occur by the quick-freezing technique of fresh excised tissues, because of anoxia and ischemia (Fig. 38.1b). To the contrary, the IVCT or cryobiopsy with FS fixation method always keeps soluble proteins in cells and tissues of living animal organ s for immunostaining (Figs. 38.1c, d and 38.2). As described above, the FS fixation method can maximize the benefits of various cryotechniques, which can usually maintain all the material components in the amorphous ice (Figs. 38.1 and 38.2).
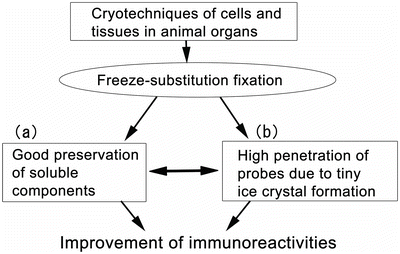

Fig. 38.2
Some technical merits of the application of cryotechniques and the following freeze-substitution fixation for immunostaining, such as molecular preservation (a) and penetration of probes (b)
38.2 Practical FS Procedures
As stated above, the FS fixation is very useful as a fixation procedure of living animal tissue samples. The FS solution is usually the organic acetone solvent , which contains paraformaldehyde at a concentration of 2 % for light microscopy . The chemical paraformaldehyde powder is first diluted at a concentration of 20 % in water, which is added with the common molecular sieve to completely remove residual water contents. Although solid osmium tetroxide crystals are directly dissolved in acetone for the conventional morphology by electron microscopy , both glutaraldehyde and paraformaldehyde are usually diluted in acetone to be used for immunostaining at a light microscopic level after the preparation of solution with their higher concentrations. The stored frozen tissue samples are taken out of the liquid nitrogen (−196 °C) into the FS solution in a container with about 5 ml capacity cooled with dry ice acetone (about −80 °C). Then, it can be sealed at the temperature of dry ice acetone. Before moving the frozen samples into the FS solution, we should loosen the lid of the container lightly, which can be then dipped into liquid nitrogen. Although the liquid nitrogen boils at first, while being subsided soon, the FS solution in the container begins to freeze from the portion in contact with its wall. Then, the container lid is opened, and the frozen tissue samples are transferred into the container. Finally, the container lid is tightly closed again, and all of them are returned into the dry ice acetone at about −80 °C. The usual time required for the FS fixation is approximately 20–40 h in the dry ice acetone at about −80 °C. The sample container will be gradually returned to room temperature after the FS fixation. After loosening slightly the lid of container first, the tissue samples are kept in a refrigerator (−20 °C) for about 2 h and then transferred into another refrigerator compartment (4 °C). In about 2 h, they are taken out and left at room temperature. The FS-fixed samples are lightly washed with pure acetone three times and embedded in paraffin wax through xylene for light microscopy. On the other hand, they are sometimes embedded in hydrophilic synthetic resin for electron microscopy with immunostaining.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


