Chapter 197 Fluoroquinolones
STRUCTURE AND PHYSICAL PROPERTIES
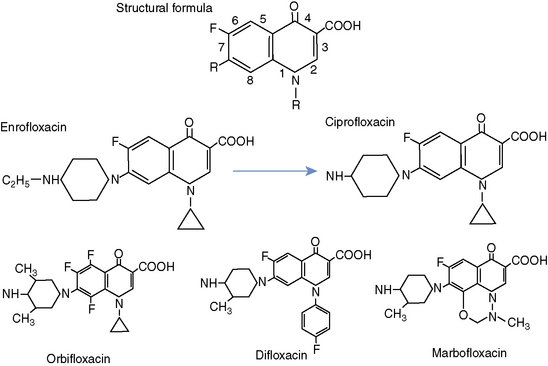
Fluoroquinolones are weak organic acids. They have amphoteric properties as a result of having an acidic group (carboxylic acid) and a basic group (tertiary amine); they are soluble in both alkaline and acidic solutions. All quinolone derivatives in clinical use have a dual ring structure with a nitrogen at position 1, a carbonyl group at position 4, and a carboxyl group attached to the carbon at the 3 position of the first ring (Figure 197-1).1
Earlier fluoroquinolones, such as nalidixic acid, did not achieve systemic antibacterial levels. As a result, these agents had limited clinical utility and were suitable only for treating lower urinary tract disease. Fortunately, several structural modifications to the original dual ring have resulted in increased potency, extended spectrum, and enhanced bioavailability. For example, the addition of a fluorine at position 6 led to increased efficacy against both gram-negative and gram-positive bacteria, and substitutions at position 7 result in increased potency and increased antipseudomonal activity. At position 8, addition of a halide, fluorine, or a methoxy group enhances activity against anaerobic bacteria (see Figure 197-1).1 A more extensive discussion of the relationships between structure and activity of the quinolone class is beyond the scope of this chapter.
SPECTRUM
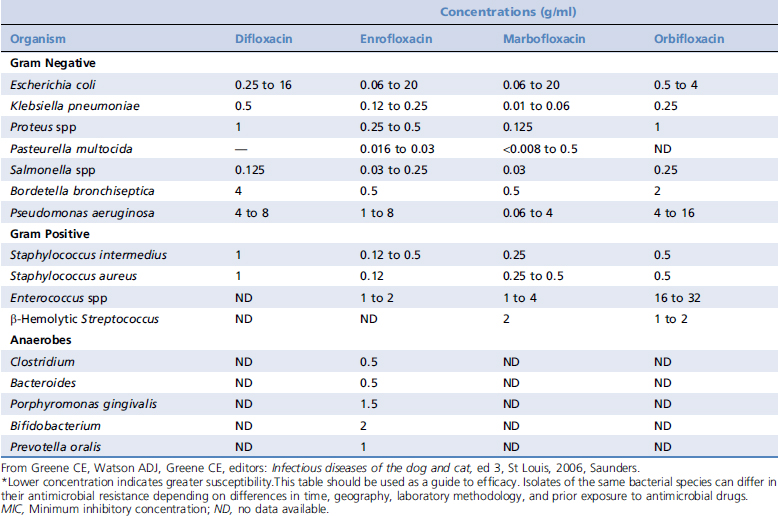
Although the fluoroquinolone antibiotics all possess the same basic chemical structure, agents within the class exhibit variability in their spectrum and potency (Table 197-1). These antibiotics differ from other antibiotics such as penicillins, tetracyclines, and macrolides in that they exhibit a high degree of efficacy at relatively low serum concentrations. In addition, minimal bactericidal concentrations of quinolones are usually within two-fold to four-fold of the minimum inhibitory concentration (MIC) for many of their target organisms.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree