Chapter 180 Myocardial disease in cats is a heterogeneous group of conditions, paralleling the spectrum of diseases that make up human cardiomyopathy. Feline cardiomyopathies originally were categorized according to morphology and function, in line with the contemporary human classification. The main categories were hypertrophic, dilated, restrictive, and unclassified cardiomyopathy, with the later addition of arrhythmogenic right ventricular cardiomyopathy (ARVC). Human cardiomyopathies now are divided into primary cardiomyopathies in which the myocardial changes are the major abnormality (e.g., hypertrophic cardiomyopathy, or HCM) and secondary cardiomyopathies in which a multiorgan systemic disease (e.g., hyperthyroidism) affects the myocardium (Maron et al, 2006). Primary cardiomyopathies are divided into those with a genetic basis, those with an acquired cause, and those involving a combination of genetic and acquired factors (mixed). Hypertrophic cardiomyopathy and ARVC are considered genetic in humans, whereas dilated cardiomyopathy (DCM) and restrictive cardiomyopathy (RCM) are classed as mixed. There is evidence that HCM can be genetic in cats: two separate mutations in the cardiac myosin-binding protein C gene have been identified in Maine coon and Ragdoll cats with HCM. A deficiency in dietary taurine can result in a feline DCM phenotype. Secondary cardiomyopathies found in cats include myocardial disease related to hyperthyroidism, systemic hypertension, and chronic anemia, which commonly affect cardiac chamber size and function. Healthy cats may have innocent heart murmurs that are not associated with any structural cardiac abnormality. The prevalence of murmurs in the healthy cat population is high, but as many as half of all feline murmurs may be functional and not actually caused by structural heart disease (Wagner et al, 2010). These low- to moderate-intensity systolic murmurs often are associated with conditions characterized by increased sympathetic tone and high cardiac output, such as stress, anemia, hyperthyroidism, and fever, with the murmur arising from the left or the right ventricular outflow tract. Dynamic right ventricular outflow tract obstruction can be normal in cats. The best way to differentiate cats with functional murmurs from those with heart disease is with echocardiography. Unfortunately, interpretation of echocardiograms in cats requires a high level of expertise, particularly in distinguishing between normal cats and cats with mild structural cardiac changes. Thoracic radiography may be used to identify cardiomegaly in asymptomatic cats, but the sensitivity of this method is low, especially in cats with mild structural changes. Plasma biomarker assays such as those for N-terminal prohormone B-type natriuretic peptide (NT-proBNP) and high-sensitive cardiac troponin I are showing promise as screening tests for cardiomyopathy in this setting but always should be used in conjunction with other tests (Fox et al, 2011). It is relatively easy to screen for secondary cardiomyopathies by measuring blood pressure, thyroxine concentration, and hematologic parameters. Cats with myocardial disease may have no detectable abnormalities on physical examination. The extent of this problem is unclear, although a recent study evaluating 103 apparently normal cats found heart murmurs in only 5 of the 16 cats with echocardiographic evidence of cardiomyopathy (Paige et al, 2009). Additionally, it is common for cats brought for treatment of aortic thromboembolism (ATE) and advanced cardiomyopathy to have shown no signs of cardiac disease at previous examinations. Without preemptive screening, these at-risk cats likely will continue to be denied the opportunity for cardiac therapy. Clearly it is never appropriate to treat a cat for heart disease based solely on the presence of an auscultatory abnormality such as a murmur or even a gallop sound or click. Most cats with heart disease have cardiomyopathy, but those with secondary cardiomyopathies must be identified because their treatment should be directed at the underlying disease. It is more important to determine symptomatic status than to try to categorize the cardiomyopathy phenotype because cats with clinical signs are managed differently from those without. Treatment decisions ideally should be evidence based, but relevant data mostly are lacking in feline heart disease. In the absence of valid evidence, management should focus on targeting cats at risk of recognized complications of cardiomyopathy. The most common sequelae of cardiomyopathy are CHF and ATE, although the incidence of sudden death is probably underestimated. When specific hemodynamic disturbances (e.g., systolic dysfunction) are identified, therapy should be tailored to the specific functional problem. Nevertheless, it is important to recognize that many cats with myocardial disease remain asymptomatic for long periods without any intervention (Payne et al, 2010), and any incremental benefit from therapy in these cats may not justify the additional expense, time, and stress. Management strategies are presented in the following sections according to risk stratification for stages of heart disease (Table 180-1). Cats with occult cardiomyopathy exhibit a spectrum of risks, from those cats that will survive to old age and never experience any clinical signs associated with their heart disease to those at imminent risk of CHF or ATE. Cats at the low-risk end of this spectrum are likely to have a normal-sized left atrium (Payne et al, 2010) and may be difficult to differentiate from healthy cats if ventricular changes are subtle. Echocardiography is the most effective test for evaluating left atrial size in cats, although radiography can provide an approximate assessment in cats with moderate or severe atrial enlargement. Alternatively, plasma NT-proBNP concentrations may be used to provide an initial risk assessment to guide further testing (Fox et al, 2011). Note that although cats with mild occult disease are unlikely to require treatment and often remain in stable condition for long periods, the risk remains that CHF will develop in response to interventions such as intravenous fluid therapy, anesthesia, or depot corticosteroid administration, or to periods of prolonged tachycardia associated with stress. Worryingly, cats in this category also may be at risk of sudden death (Payne et al, 2011). Although ACE inhibitors have theoretic benefits in cardiomyopathy, there are no data supporting their use in asymptomatic cats. Well-controlled studies in asymptomatic Maine coon cats failed to show a benefit of 12 months of treatment with ramipril at 0.5 mg/kg q24h PO (MacDonald et al, 2006). Another randomized, controlled study (Taillefer and Di Fruscia, 2006) comparing the effects of benazepril (0.5 mg/kg q24h PO) and diltiazem CD (10 mg/kg q24h PO) in 21 cats with preclinical HCM did not identify any relevant difference between groups after 6 months, although a control group was not studied. However, some cardiologists do consider an ACE inhibitor for treatment of asymptomatic cats demonstrating moderate and severe left atrial enlargement. Although severe left ventricular hypertrophy has been identified as an independent risk factor in people with HCM, and thus therapeutic targeting of left ventricular hypertrophy has been suggested, similar information is not available in feline HCM. Moreover, the mechanisms of hypertrophy may be so diverse in different forms of HCM that one common therapeutic strategy may not be capable of achieving the desired result. ACE inhibitors or angiotensin II receptor antagonists, spironolactone, diltiazem, and statins all have been used successfully in experimental HCM models. However, neither the ACE inhibitor regimen in the study cited previously nor 4 months of treatment with the aldosterone antagonist spironolactone (2 mg/kg q12h PO) showed any effect on estimates of left ventricular diastolic function or chamber dimensions in cats with preclinical HCM (MacDonald et al, 2006, 2008). In the spironolactone study, several cats developed skin lesions with chronic administration. The specific risk of ATE in asymptomatic cats with HCM has not been reported. Most clinicians view left atrial enlargement as a likely risk factor for this complication, and many consider antithrombotic therapy appropriate in cats with HCM with this echocardiographic finding. This treatment is discussed in the following paragraphs and in Chapter 181. Evaluation of size, content, and flow velocity in the left auricle may contribute to the decision on whether to use antithrombotic treatment (Schober and Maerz, 2006).
Feline Myocardial Disease
Definition of Myocardial Disease
Diagnostic Approach
Therapeutic Approach
Asymptomatic Cardiomyopathy (Low Risk)
Asymptomatic Cardiomyopathy (at Risk)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


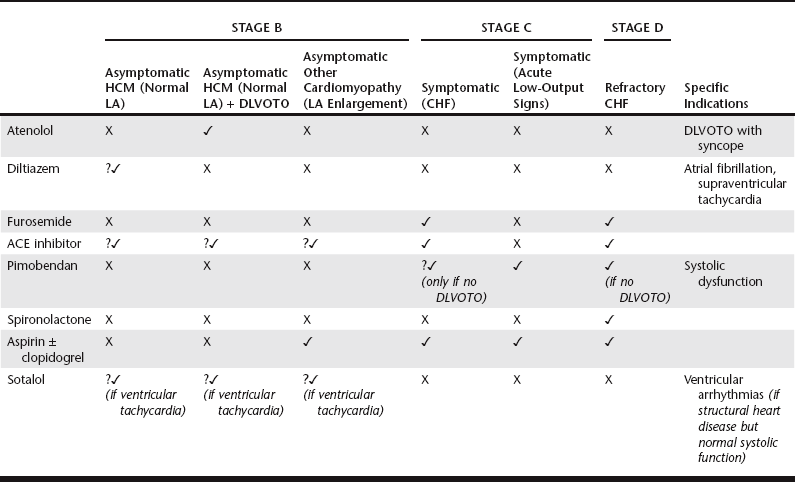
 , used by the authors; ?, greater degree of uncertainty; ACE, angiotensin-converting enzyme; CHF, congestive heart failure; DLVOTO, dynamic left ventricular outflow tract obstruction; HCM, hypertrophic cardiomyopathy; LA, left atrium.
, used by the authors; ?, greater degree of uncertainty; ACE, angiotensin-converting enzyme; CHF, congestive heart failure; DLVOTO, dynamic left ventricular outflow tract obstruction; HCM, hypertrophic cardiomyopathy; LA, left atrium.