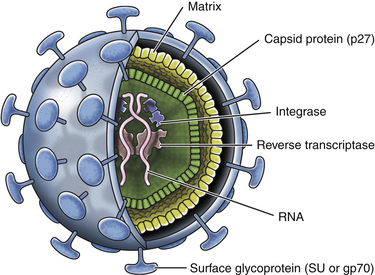
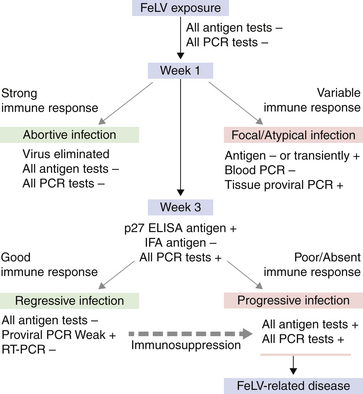
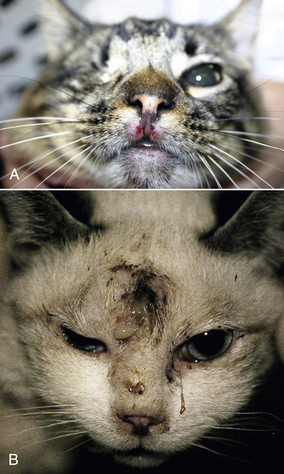
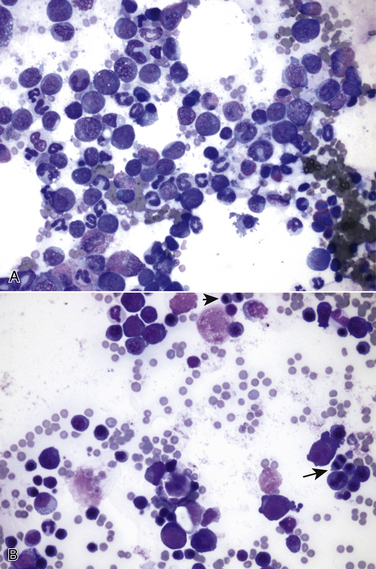
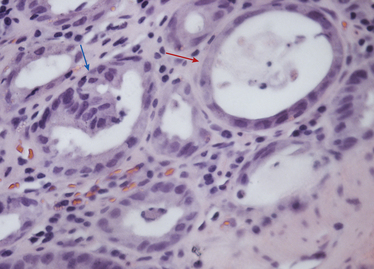
Chapter 22 FeLV is an enveloped RNA virus that belongs to the genus Gammaretrovirus of the family Retroviridae. FeLV infection remains an important cause of mortality in domestic cats through its ability to cause immune suppression, bone marrow disorders, and hematopoietic neoplasia. FeLV also causes disease in wild felids such as the highly endangered Iberian lynx.2 The structure of FeLV is similar to that of FIV, except that the capsid is icosahedral rather than cone shaped (Figure 22-1). There are three main subtypes of FeLV: FeLV-A, FeLV-B, and FeLV-C. Each subtype uses a different receptor to enter cells (Table 22-1). All cats infected with FeLV-B and FeLV-C are co-infected with FeLV-A, and only FeLV-A is transmitted between animals. FeLV-B and FeLV-C are more pathogenic than FeLV-A. FeLV-B arises through recombination of FeLV-A proviral DNA with endogenous FeLV sequences present in host cellular DNA.3 FeLV-C arises from accumulation of mutations or insertions in the env (SU) gene of FeLV-A.4,5 The FeLV subtype influences the clinical expression of disease (see Table 22-1). For example, FeLV-C is associated with nonregenerative anemia. Even within an FeLV subtype, mutations in the SU and the LTR regions of the viral genome affect disease outcome.6,7 An additional subtype, FeLV-T, has been associated with immunodeficiency. TABLE 22-1 Host Cellular Receptors Involved in FeLV Infection ∗Mendoza R, Anderson MM, Overbaugh J. A putative thiamine transport protein is a receptor for feline leukemia virus subgroup A. J Virol 2006;80(7):3378-3385. †Anderson MM, Lauring AS, Robertson S, et al. Feline Pit2 functions as a receptor for subgroup B feline leukemia viruses. J Virol 2001;75(22):10563-10572. ‡Keel SB, Doty RT, Yang Z, et al. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science 2008;319(5864):825-828. FIGURE 22-1 Structure of feline leukemia virus. Gammaretroviruses contain two identical strands of RNA and associated enzymes, which include reverse transcriptase, integrase, and protease, packaged into a core composed of the capsid protein (p27) with a surrounding matrix, all enclosed by a phospholipid membrane envelope derived from the host cell. The envelope contains a gp70 glycoprotein and the transmembrane protein p15E. Transmission of FeLV-A primarily results from close contact with salivary secretions, such as through licking, mutual grooming, and shared food and water dishes. Other routes of transmission, such as by biting, blood transfusion, in milk, and possibly by fleas, can also occur.8–10 The virus survives poorly outside the cat and is readily inactivated by disinfectants, soap, and desiccation. The overall prevalence of FeLV infection has declined over the past two decades with more extensive testing and vaccination. Before the institution of widespread testing and vaccination, more than 30% of cats in some catteries were infected.11 In the early 1990s, the overall prevalence of infection was 13% in nearly 28,000 sick cats from North America that were at risk for exposure.12 The prevalence was just 7% in a similar population of approximately 1400 cats in 2006.13 The prevalence of FeLV infection in European cats has also declined markedly.14 Currently, the overall prevalence of infection in mixed populations of cats is 1% to 6%.13,15–19 Cats with access to the outdoors; those that have contact with other cats; cats that are male, aggressive, or intact; and cats that are co-infected with FIV are at increased risk of FeLV infection.12,13,15,17 Male cats are less strongly predisposed to FeLV infection than to FIV infection, and in some studies,20 no male predisposition has been recognized. Adult cats are more likely to be infected with FeLV than cats aged less than 6 months,13,17 but the median age of cats infected with FeLV is 3 years,15 which is lower than that for FIV. This reflects a) the greater degree of pathogenicity of FeLV than FIV and its ability to significantly reduce lifespan; and b) the phenomenon of age-related resistance to FeLV, whereby exposure of cats that are less than 4 months of age to the virus is much more likely to lead to progressive infection than exposure of adults.21 When 12- to 16-week old kittens are exposed to a multicat household with endemic FeLV infection, between 60% and 70% of kittens will become infected within a 5-month time period. In contrast, less than 5% of cats aged 6 months or older will become infected over the same time period; over a 2-year period, 40% to 50% of these adult cats will become infected. Infection of adult cats can also still occur with exposure to high doses of the virus or when there is underlying host immune compromise. Possible outcomes of infection with FeLV are shown in Figure 22-2. The immune system of some infected cats suppresses viral replication within a few weeks after infection, before significant infection of the marrow occurs. These cats develop a regressive infection, whereby proviral DNA is present in the host cell genome but production and shedding of virus no longer occurs (see Chapter 21). This may occur after the initial period of viremia, or viremia may never be detectable.22 Regressive infection can persist for life and may be reactivated with immunosuppression, such as might occur during pregnancy or following treatment with immunosuppressive drugs.23 Later in life, an unknown percentage of cats with regressive infections may develop FeLV-negative malignancies as a result of integration of viral DNA within host cellular oncogenes. Most cats with regressive infection, however, never develop clinical signs related to FeLV infection. Viral genome sequences may eventually be incompletely replicated, and as a consequence, reactivation of virus replication will become impossible in some cats over time. In abortive infections, no viremia occurs after infection, and virus cannot be detected using any method. Cats with abortive infections have been exposed to low doses of FeLV, and although they fail to develop viremia, they will develop antibodies to the virus.24 Some cats that never test antigen-positive may have focal infections, that is, evidence of proviral DNA in some tissues but not in blood or bone marrow.9 Cats develop progressive infection once involvement of the marrow is established and cellular destruction by the virus exceeds the ability of the host’s immune system to suppress viral replication. Persistent viremia and progressive FeLV-related disease result (Box 22-1). FIGURE 22-2 Outcomes of infection with FeLV. In the first week of infection, cats test negative with all antigen assays (i.e., p27 ELISA and IFA assays), RT-PCR for viral RNA, and PCR for proviral DNA. Rarely, cats exposed to low levels of virus eliminate the infection before or at this time point (abortive infection). Soluble antigen assays and proviral PCR assays become positive from about the third week after infection, and virus is shed in saliva, tears, urine, and feces. After this time point, productive viral infection is suppressed by the host immune response (regressive infection) or the virus multiplies rapidly in the bone marrow and a progressive infection occurs, which is characterized by persistently positive antigen ELISA, IFA, viral RNA, and proviral DNA assay results. Cats with focal or atypical infection are never or transiently antigen positive and test negative using PCR assays on blood or bone marrow, but some tissues (such as the mammary gland or bladder) are positive with proviral PCR. The latter occurs in only a small percentage (e.g., < 5%) of infected cats. Opportunistic infections may develop in FeLV infections as a result of myelo suppression or an acquired cell-mediated immunodeficiency. The immunosuppressive properties of FeLV are not fully understood but have been linked in part to the viral envelope peptide, p15E, which inhibits T and B cell function, inhibits cytotoxic lymphocyte responses, alters monocyte morphology and distribution, and has been associated with impaired cytokine production and responsiveness.25–28 Kittens with progressive FeLV infection have impaired T cell and, to a lesser extent, B cell function.29–32 Infected cats may develop lymphopenia, thymic atrophy, and depletion of lymphocytes within lymph node paracortical zones. CD4+ T cell malfunction may contribute to a decreased humoral and cell-mediated immune response in affected cats,33,34 and the response to vaccination may also be impaired. Impaired neutrophil function compounds the effect of neutropenia in some infected cats.35–37 Opportunistic infections that result include bacterial infections of the upper and lower urinary tract, hemoplasmosis, upper respiratory tract infections, feline infectious peritonitis, chronic stomatitis, toxoplasmosis, dermatophytosis, and cryptococcosis (Figure 22-3). The prevalence of some of these infections in cats with FeLV infection does not differ significantly from that in cats not infected with FeLV, but clinical signs are more severe and refractory to therapy. Infection with FeLV is an established risk factor for hemoplasma infection. In one study, it was also a risk factor for Bartonella henselae infection, but no evidence of clinical disease was identified in the cats infected with B. henselae.38 FIGURE 22-3 Opportunistic infections in cats with progressive FeLV infection. A, Ulceration of the nasal planum in a 6-month-old male neutered domestic medium hair cat with progressive FeLV infection as defined by positive ELISA antigen and IFA assays on peripheral blood. Stomatitis was also present, and feline calicivirus infection was suspected. B, Severe nasal cryptococcosis in a Siamese cat from a cattery with endemic FeLV infection. FeLV causes neoplasia in cats primarily as a result of insertional mutagenesis, by which the virus activates proto-oncogenes (especially c-myc, but also others such as flit-1) or disrupts tumor suppressor genes.39 The most common types of neoplasia in cats infected with FeLV are lymphoma and leukemia. Cats infected with FeLV are more than 60-fold more likely to develop lymphoma than cats not infected with FeLV; this compares with approximately 5-fold for FIV.40 Lymphoma or leukemia can be detected in nearly one quarter of cats with progressive FeLV infection at necropsy.41 In the 1980s, as many as 70% of cases of feline lymphoma were associated with FeLV infection, but with improved control measures and vaccination, the vast majority of cats (more than 80% to 90%) seen at veterinary clinics with lymphoma now test negative for FeLV antigen.42–44 The most common types of lymphoma in cats infected with FeLV are thymic (mediastinal), multicentric, spinal, renal, or ocular lymphoma. FeLV-associated lymphomas are mostly of T-cell origin, but B cell lymphoma can also occur. In contrast, FIV-associated lymphomas tend to be of B-cell origin.42 Approximately 80% of cats with thymic lymphoma test positive for FeLV antigen, whereas fewer than 10% of cats with gastrointestinal lymphoma are FeLV antigen-positive. Large granular lymphoma is rarely associated with FeLV infection.45 Cats with thymic lymphoma typically develop clinical signs of lethargy, tachypnea, and sometimes regurgitation. Most FeLV-positive cats with lymphoma are less than 4 years of age.14 Cats with regressive infection appear to be at greater risk for development of lymphoma than cats that were never exposed to FeLV. FeLV-negative cats that live with cats that test positive for FeLV antigen have a more than 40-fold increased risk of lymphoma compared to that expected without exposure to FeLV.46 Several studies have investigated the prevalence of FeLV proviral DNA in tumor cells from cats that test negative for FeLV antigen. Some studies (including more recent studies that used real-time PCR assays) found no evidence of FeLV proviral DNA in feline lymphomas.14,47,48 In older studies that used conventional PCR, more than 20% of antigen-negative lymphomas tested positive.49,50 Additional studies from a variety of geographic locations that use multiple real-time PCR assays are required to clarify the role that FeLV plays in lymphomas and leukemias that develop in cats that test negative for FeLV antigen. FeLV is responsible for the majority of myelogenous leukemias, erythroleukemias, megakaryocytic, and lymphoid leukemias in cats, although not all cats with these tumors test positive for FeLV antigen. Most FeLV-associated leukemias are acute. FeLV infection can underlie chronic eosinophilic leukemia,51 chronic myelomonocytic leukemia,52 and chronic lymphocytic leukemia. However, most cats with chronic lymphocytic leukemia are seronegative for FeLV antigen. FeLV infection can result in the development of multiple fibrosarcomas in young cats. These occur when FeLV-A recombines with cellular oncogenes (such as c-fes, c-fms, or c-fgr) to form feline sarcoma viruses (FeSV). These viruses then develop mutations in these oncogenes that, when reinserted into cellular DNA, cause malignant transformation.53,54 To replicate, FeSV requires the presence of FeLV-A, which supplies proteins such as those encoded by the env gene. Thus, all cats infected with FeSV test positive for FeLV antigen. FeSV-associated fibrosarcomas are characterized by multifocal, locally invasive, often ulcerated cutaneous masses that metastasize readily to the lung and other sites with a poor prognosis. FeLV-FeSV DNA sequences have been detected in some uveal melanomas from cats,55 but other studies have failed to identify an association between FeSV and ocular melanomas or sarcomas in cats.56,57 FeLV infection does not appear to be important in the pathogenesis of solitary fibrosarcomas or injection-site sarcomas in cats.58,59 Other tumor types that are variably associated with FeLV infection are feline olfactory neuroblastomas, cutaneous horns, and osteochondromatosis (multiple cartilaginous exostoses).60 Feline olfactory neuroblastomas are rare and aggressive tumors of the nasal cavity that can lead to signs of rhinosinusitis and neurologic signs. Osteochondromatosis is characterized by benign cartilage-capped exostotic growths that arise from the surface of a bone, which can cause pain, lameness, disfigurement, and paresis due to spinal cord compression. Cutaneous horns occur as a result of benign keratinocyte hyperplasia. Multiple mechanisms can lead to anemia in cats infected with FeLV (Box 22-2). Approximately 90% of FeLV-associated anemias are nonregenerative.61 A variety of bone marrow disorders lead to decreased red blood cell production. FeLV-C infection results in pure red cell aplasia, a severe nonregenerative anemia associated with erythrocyte macrocytosis and depletion of erythroid precursors in the bone marrow. This occurs because FeLV-C binds to and interferes with a heme exporter protein, which results in subsequent heme toxicosis to the developing erythrocyte.62 The presence of macrocytosis in the absence of reticulocytosis should thus raise suspicion for FeLV infection. FeLV infection can also lead to aplastic anemia in some cats, which is a deficiency in all cell lineages (platelets, myeloid, and erythroid) in the bone marrow, and replacement of the bone marrow space by adipose tissue. Anemia and bone marrow dysfunction may also result from myelophthisis secondary to leukemia, myeloid and/or erythroid dysplasia, or myelofibrosis (progressive replacement of the marrow with collagenous connective tissue) (Figure 22-4). Myelodysplasia is characterized by disordered maturation of marrow precursors, which in some cases precedes emergence of leukemia. FeLV infection underlies many myelodysplastic syndromes (MDS) and leukemia in cats.63 However, over the past two decades, 50% of 28 cats with myelodysplasia and 44% of 41 cats with leukemia seen at the University of California, Davis, tested negative for FeLV antigen in peripheral blood, and only 36% of 34 cats with dysmyelopoiesis seen at the University of Minnesota were positive for FeLV antigen.64 Several different classification systems have been used to describe feline MDS.64 The French-American-British (FAB) classification scheme used for humans has been modified for dogs and cats. This divides MDS into 2 groups: 1) MDS with refractory cytopenia (less than 6% myeloblasts in the bone marrow, MDS-RC) and 2) MDS with excessive numbers of myeloblasts (6% to 30% myeloblasts, MDS-EB). The presence of more than 30% blasts indicates leukemia. The most common form of MDS in cats is MDS-EB.64 Regressive FeLV infections have been detected by proviral DNA PCR in some cats with bone marrow disorders,65 but the relationship between the presence of proviral DNA and the pathogenesis of disordered marrow function is unclear. FIGURE 22-4 Bone marrow abnormalities in cats with FeLV infection. A, Bone marrow aspirate cytology from a 6-month old male neutered domestic shorthair cat that was evaluated for lethargy and fever. Acute leukemia, marked erythroid aplasia, marked granulocytic dysplasia, and increased eosinophilopoiesis is present. Megakaryocytic dysplasia was also identified. The G:E ratio was approximately 2950:1 with marked erythroid aplasia. Wright’s stain. B, Bone marrow aspirate cytology from a 1-year-old male neutered domestic shorthair. Severe megaloblastic erythrodysplasia and mild megakaryocytic dysplasia were identified. The myeloid series is present but markedly decreased in all stages; the myeloid to erythroid ratio was estimated at 1:6.5 (compare with A). Nuclear to cytoplasmic asynchrony (megaloblastic change) is present, with immature nuclei in cytoplasm with hemoglobinization. Mitotic figures are present in moderate numbers and are seen in the late stages of the erythroid series (arrows). Anemia in cats with FeLV infection may also result from anemia of inflammatory disease, which can be triggered by opportunistic infections or neoplastic disease. Erythrocyte destruction occurs in some FeLV-infected cats as a result of secondary immune-mediated hemolytic anemia (IMHA) or co-infection with hemoplasmas (see Chapter 41). Hemorrhage as a result of thrombocytopenia or defective platelet function may also contribute to anemia. Mechanisms of thrombocytopenia include immune-mediated thrombocytopenia (ITP) and bone marrow dysfunction. Similarly, neutropenia can result from myeloid hypoplasia, myelofibrosis, myelodysplasia, myelophthisis, or maturation arrest at the myelocyte or metamyelocyte stages. In some cases, peripheral neutropenia accompanied by myeloid hyperplasia reflects underlying immune-mediated neutropenia. In addition to immune-mediated cytopenias, other immune-mediated disorders that can occur in association with progressive FeLV infection are glomerulonephritis,66 uveitis,67 and polyarthritis.68 Circulating immune complexes have been detected in infected cats.69–71 Because primary immune-mediated disorders are otherwise rare in cats, retrovirus testing is essential in cats that are diagnosed with these conditions before immunosuppressive drug treatment is initiated. Neurologic disorders in cats with progressive FeLV infections may occur secondary to central nervous system (CNS) neoplasia, opportunistic infections, or FeLV infection itself. The envelope protein of FeLV may be neurotoxic.72,73 Anisocoria, mydriasis, Horner’s syndrome, and urinary incontinence can occur. FeLV infection may be one of the most frequent causes of urinary incontinence in cats, an otherwise uncommon condition. A myelopathy has been described in FeLV-infected cats that showed various neurologic signs such as disorientation, lethargy, increased vocalization, progressive ataxia, paresis, paralysis, hyperesthesia, urinary incontinence, recurrent constipation, and anisocoria with diminished pupillary light reflexes.74 At necropsy, abundant FeLV antigen was detectable within neurons, endothelial cells, oligodendroglia, and astrocytes within the spinal cord.74 Uncommonly, FeLV causes enteritis that clinically and histologically resembles that caused by feline panleukopenia virus (FPV), except that lymphoid depletion is absent.75 Clinical signs include vomiting, acute or chronic diarrhea that may be hemorrhagic, inappetence, weight loss, and dehydration. FeLV-infected cats with “panleukopenia-like syndrome” have intestinal crypt destruction and pancytopenia that results from myeloid destruction (Figure 22-5).76 Although now very rare, co-infections with FeLV and FPV can also occur, and the 2 viruses may enhance each other’s pathogenicity.77 Co-infections with FeLV and other enteric viruses, such as feline coronavirus, also occur.78 In most cases, diarrhea in cats infected by FeLV results from an etiology other than FeLV alone. FIGURE 22-5 Histopathology of a colonic biopsy specimen from a 6-year-old intact male FeLV+ domestic shorthair with a 1-week history of anorexia, weight loss, lethargy, fever, and bloody mucoid diarrhea. Severe, diffuse, plasmacytic colitis was present with crypt epithelial necrosis and regeneration. One crypt is dilated, lined with attenuated epithelium, and contains necrotic debris (red arrow). An adjacent crypt shows evidence of regeneration (blue arrow). A tissue gram stain showed a mixed population of bacteria on the luminal surface, but not within crypts. A CBC showed thrombocytopenia (22,000 platelets/µL) and neutropenia (2700 neutrophils/µL) with neutrophil toxicity. A fecal parvovirus antigen test was negative, and bone marrow aspirate cytology showed evidence of myelodysplasia. Remarkably, this cat lived another 3 years with transfusion support, but ultimately was euthanized because of acute granulocytic leukemia with secondary sepsis. Transplacental spread of FeLV can lead to fetal resorption, abortion, neonatal death, and fading kitten syndrome.76 Fetal loss may also result from secondary endometritis. Kittens infected in late gestation or after birth develop thymic atrophy, failure to nurse, dehydration, lethargy, and death within the first 2 weeks of life.
Feline Leukemia Virus Infection
Etiology and Epidemiology
Clinical Features
Opportunistic Infections
Neoplasia
Anemia and Bone Marrow Disorders
Immune-Mediated Disorders
Neurologic Disorders
Gastrointestinal Disease
Reproductive Disorders
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Feline Leukemia Virus Infection
Only gold members can continue reading. Log In or Register to continue
