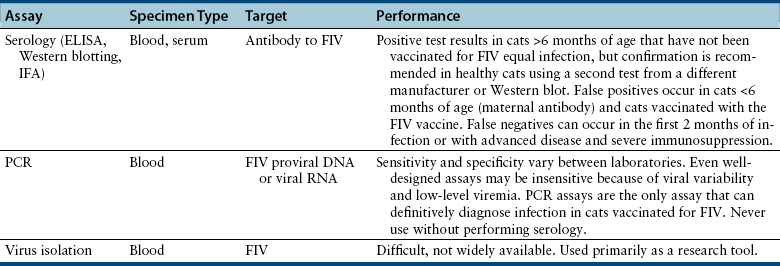
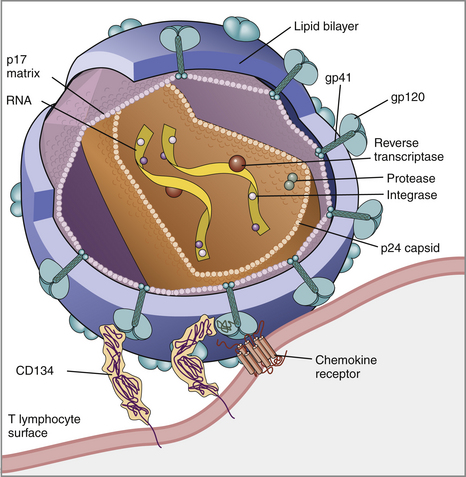
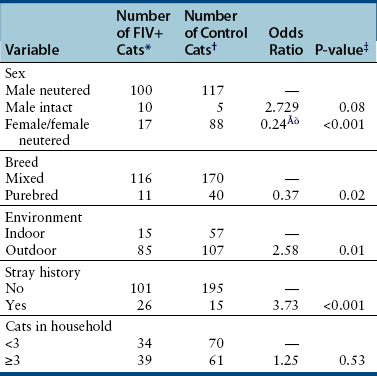
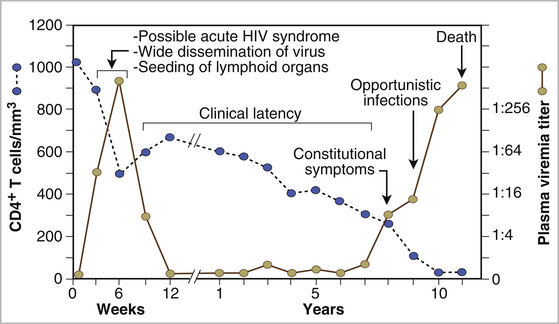
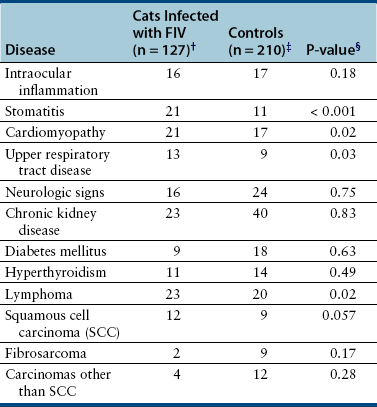
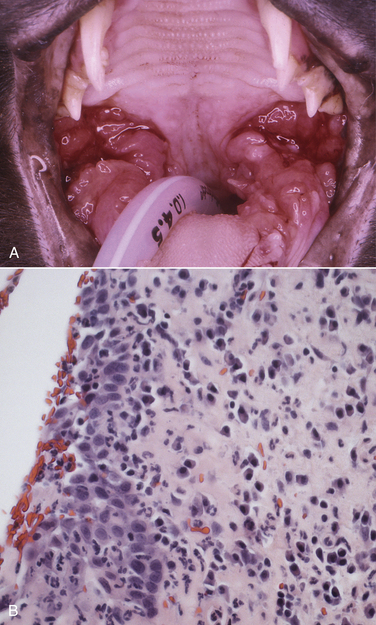
Chapter 21 Feline immunodeficiency virus (FIV) is an enveloped, RNA virus that belongs to the Lentivirus genus of the Retroviridae. It infects domestic and wild cats worldwide, as well as hyenas.4 Like HIV, FIV establishes a chronic, persistent infection that, in some cats, ultimately culminates in immunodeficiency. Because of its similarities to HIV, FIV infection in cats has been used as a research model for HIV infection and acquired immunodeficiency syndrome (AIDS),5 and relative to other viral infections of companion animals, much is known about its pathogenesis. Knowledge of retroviral structure and replication is required in order to understand diagnostic, treatment, and prevention strategies that target these viruses. Like other retroviruses, FIV has a three-layered structure that is composed of an innermost genome-nucleocapsid complex with helical symmetry, an icosahedral capsid, and an envelope with glycoprotein spikes (Figure 21-1). The FIV genome contains three major genes: gag, which encodes the virion core proteins (capsid [p24], nucleocapsid, and matrix); pol, which encodes the reverse transcriptase, protease and integrase enzymes; and env, which encodes surface (gp120) and transmembrane virion (gp41) envelope glycoproteins. Several other accessory and regulatory genes are also present. FIV invades cells via the primary receptor CD134, which is expressed on feline CD4+ T cells, B cells, and activated macrophages,6,7 and the secondary receptor CXCR4, which is normally a chemokine receptor. The viral envelope fuses with the cell membrane, and the capsid enters the cytoplasm, where reverse transcription occurs and a double-stranded DNA (dsDNA) copy of the retroviral genome is made. Additional sequences, known as long terminal repeats (LTRs), are added to either end of the viral genome. The virus then passes into the nucleus where the dsDNA copy integrates into the host genome; at this point the integrated dsDNA becomes a provirus. Transcription of this DNA, which is controlled by the LTRs, leads to synthesis of new virion components, and virus assembly and budding occur at the cell surface (Figure 21-2). Depending on the cellular environment, proviral DNA may either be latent, whereby transcription does not occur, or be transcriptionally active. Latency is one mechanism by which retroviruses can evade the host immune system. Because the reverse transcriptase enzyme is prone to error, the mutation rate of retroviruses is high, and a diversity of viral variants continuously emerges from infected hosts. Integration of retroviral DNA into host cell DNA can disrupt genes that are responsible for cell growth and differentiation (proto-oncogenes). Alternatively, cellular oncogenes captured and carried by retroviruses develop mutations during retroviral replication, and are then re-inserted into host cell DNA. The end result is abnormal cell growth and differentiation, which results in tumor formation by some retroviruses. FIGURE 21-1 Structure of FIV. An FIV virion is shown adjacent to the surface of a CD4+ T cell. Lentiviruses contain two identical strands of RNA (the viral genome) and associated enzymes, which include reverse transcriptase, integrase, and protease, packaged into a core composed of the p24 capsid protein with a surrounding p17 protein matrix, all enclosed by a phospholipid membrane envelope that is derived from the host cell. Viral encoded membrane proteins (gp41 and gp120) are bound to the envelope. CD134 and CXCR4 (chemokine) receptors on the host cell surface function as the receptors for FIV. FIGURE 21-2 The life cycle of FIV. The sequential steps in FIV reproduction are shown, from initial infection of a host cell to release of a new virus particle. An infected cell produces many virions, each capable of infecting nearby cells, with subsequent spread of the infection. Based on sequence diversity of the env gene, there are six different subtypes of FIV, A through F. Subtypes A and B are distributed most widely, followed by subtype C8–10—although a recent study from the United States showed an equal distribution of subtypes A, B, and C (Table 21-1).11–31 In addition, a number of recombinant subtypes have been recognized, such as A/B, A/C, B/D, B/E, and A/B/C recombinants, and additional subtypes also likely exist.9,11 The heterogeneity of the virus complicates the design of molecular diagnostic tests and vaccines for FIV. Whether differences in the clinical manifestations of disease relate to infection by different subtypes requires further study.9,10,31 TABLE 21-1 Distribution of FIV Subtypes Worldwide Retroviruses survive only minutes outside the host and are very susceptible to disinfection. FIV is shed in high concentrations in saliva, and the major mode of transmission is through bites. Transplacental transmission, transmission during parturition, and through milk have been documented experimentally, but these modes appear to be uncommon in naturally infected cats, and kittens infected by this route may not sustain productive infection.32,33 Venereal transmission has not been demonstrated, but FIV can be recovered from semen, and experimental inoculation of FIV into the vaginas of queens results in transmission.34,35 Transmission also has the potential to occur through blood donation. Seropositivity to FIV (which is equivalent to infection because of viral persistence) is consistently associated with a history of bite wounds, older age, male sex, illness, and outdoor access (Table 21-2).12,36–41 Being of mixed breed has also been a risk factor in some studies. The mean age at diagnosis is around 6 to 8 years, and 80% to 90% of cats are more than 2 years of age. Male cats are up to 4.7 times more likely to be seropositive than female cats.40 Indoor housing decreases transmission but does not eliminate it.42 Worldwide, the seroprevalence of FIV in domestic pet cats ranges from around 1% to 12%.9,40,41 A study that included more than 18,000 North American cats estimated the overall prevalence of FeLV and FIV infections as around 2.3% and 2.5%, respectively.5 Higher prevalences are found in feral and free-ranging cats and sick cats. In the North American study, the prevalence of FIV infection in sick, feral cats was 18.2%, whereas that in healthy indoor cats was only 0.7%. A study from France showed a seroprevalence of 21% in unowned cats, compared with 10% in owned cats.43 In Japan, the overall seroprevalence is very high (23% in one study), and as many as one-third of male cats test positive.12 Occasionally, co-infections with FeLV occur, and infection with one retrovirus is a risk factor for infection with the other.36,37 TABLE 21-2 Risk Factors for FIV Infection in Cats Seen at the UC Davis VMTH ∗All cats were >6 months of age and none had a history of FIV vaccination. †All control cats were negative for FIV antibody at their visit to the University of California, Davis. ‡P-values <0.05 were significant. ÅòIn other words, female cats were four times less likely to be FIV+ than male neutered cats. Modified from Trott KA, Kass PH, Sparger EE, et al. A clinical case control study: clinical presentation of FIV-positive cats. University of California, Davis, STARS in Science Day. 2007; abstr. Three phases of disease have been delineated, acute (primary), subclinical, and terminal, although not all phases are recognized in many naturally infected cats. After inoculation, the virus replicates in lymphoid tissues, and high concentrations of virus are present in blood 2 weeks after infection. A peak of viremia occurs 8 to 12 weeks after infection (Figure 21-3). There is a decline in CD4+ and CD8+ T cells in peripheral blood. This may be associated with transient illness, which lasts 3 to 6 months and is often unrecognized by cat owners. Some cats show lethargy, fever, anorexia, diarrhea, stomatitis, weight loss, and/or lymphadenopathy during the acute phase. Lymphadenopathy results from lymphoid hyperplasia, and can persist for weeks to months. Neutropenia can also occur,44 possibly as a result of neutrophil apoptosis. CD4+/CD25+ T regulator (Treg) cells are infected and activated during the acute phase. These cells then inhibit the proliferation of activated CD4+ and CD8+ T cells, and cause them to undergo apoptosis. This may contribute to persistence of FIV and further immunosuppression.45–47 Altered dendritic cell function may also occur.48,49 In general, impaired T cell function in acute infection is thought to result from cytokine dysregulation, immunologic anergy (failure to respond to specific antigens), and increased apoptosis.45 Nevertheless, most cats survive this phase because of a rebound in CD8+ T cell numbers and a strong humoral immune response. FIGURE 21-3 Changes in virus load and CD4+ T Cell numbers over the course of infection with HIV. A similar clinical course of infection occurs for FIV infection. Viremia is detected early after infection and may be accompanied by systemic signs (acute phase). Plasma viremia then falls to very low levels and remains this way for many years (subclinical phase). Although this period is referred to here as “clinical latency,” the virus itself is not latent during this time, because there is ongoing production of virus and a steady decline in CD4+ T cell counts. In the terminal phase of infection, signs of immunodeficiency develop and plasma viremia increases. Some infected cats never reach this phase. Antibody production declines, and antibody tests may be negative in cats with advanced terminal-phase disease, but PCR assays are more likely to be positive. In the subclinical (or asymptomatic) phase, CD4+ T cell numbers rebound, and the plasma virus load declines to very low levels. Cats remain subclinically infected, often for years or even for life. This is not latent infection, because virus production continues at low levels; a slow, progressive decline in CD4+ T cell numbers; reduction in the CD4+:CD8+ T cell ratio; and in some cats, hyperglobulinemia, which results from B cell hyperactivation. Some studies also describe a sustained increase in CD8+ T cell numbers. Although activated, paradoxically, T cells have a reduced ability to respond to antigenic stimulation. Altered lymphocyte expression of cell surface molecules (including CD4 and cytokine receptors, and MHC II antigens), and continued alteration of dendritic cell and neutrophil function also contribute to immunosuppression. Dysregulation of cytokine production occurs. For example, cats that are chronically infected with FIV fail to produce Il-2, Il-6, and Il-12 in response to Toxoplasma gondii infection and instead produce elevated levels of the antiinflammatory cytokine Il-10.45,50 There is a slow influx of immune cells into the brain, with gradual progression of central nervous system (CNS) disease.51 The rate of progression of the subclinical phase depends on factors such as the virus strain, co-infections with other agents that activate virus transcription, and host immunity. In some cats, these changes ultimately lead to the terminal phase, which is characterized by clinical signs of opportunistic infections, neoplastic disease, myelosuppression, and neurologic disease. This is the phase most commonly recognized in naturally infected cats (Table 21-3). However, many infected cats never develop FIV-related clinical signs, even when CD4+ T cell counts are low, and instead die from other causes. The terminal phase of FIV infection is commonly associated with moderate to severe periodontal disease, lymphoplasmacytic stomatitis (Figure 21-4), gingivitis, and feline odontoclastic resorptive lesions,52 which may result from opportunistic bacterial and viral infections. Other opportunistic infections include chronic bacterial skin and ear infections, persistent viral upper respiratory tract infections, dermatophytosis, mycobacterial infections, fungal infections such as cryptococcosis and sporotrichosis, hemoplasmosis, toxoplasmosis, and/or parasitic infections such as demodecosis and severe flea burdens. With the exception of stomatitis, the relationship between many of these opportunistic infections and FIV infection is somewhat unclear, because the prevalence of many of these infections in cats with FIV infection is similar to that in cats without FIV infection. However, infections are often more severe and less responsive to treatment than the same infections in immunocompetent cats. TABLE 21-3 Disease Diagnoses in 127 Cats with FIV Infection and 210 Age-Matched Control Cats Seen over the Same Time Period at the UC Davis VMTH∗ ∗Results should be interpreted with caution because the data were retrospectively collected. Some cats may have had undiagnosed disease, such as subclinical chronic kidney or cardiac disease. The pathogenesis of a disease process in FIV-positive cats might also differ from that found in FIV-negative cats. †All cats were >6 months of age and none had a history of FIV vaccination. ‡All control cats were negative for FIV antibody at their visit to the University of California, Davis. §As determined by chi-square (univariate) analysis. P-values <0.05 were significant. Modified from Trott KA, Kass PH, Sparger EE, et al. A clinical case control study: clinical presentation of FIV-positive cats. University of California, Davis, STARS in Science Day. 2007; abstr. FIGURE 21-4 severe lymphoplasmacytic stomatitis in a cat infected with feline immunodeficiency virus. A, There is marked hyperemia, ulceration, and proliferative lesions in the palatoglossal folds. (From Bonello D, Roy CG, Verstraete FJM. Non-neoplastic proliferative oral lesions. In: Verstraete FJM, Lommer MJ, eds. Oral and Maxillofacial Surgery in Dogs and Cats. Philadelphia, PA: Saunders; 2011.) B, Histopathology of a biopsy from a cat with severe stomatitis associated with FIV infection. The development of neoplasia in FIV-infected cats is thought to result primarily from immune suppression, although there is serologic evidence that cats may be infected with a gammaherpesvirus similar to Epstein-Barr virus that might be reactivated in cats infected with FIV.53 Lymphomas are the most commonly reported FIV-associated tumor, especially B cell lymphomas, but also T cell and non-B, non-T cell lymphomas.54 FIV-infected cats are 5 times more likely to develop lymphoma than cats not infected with FIV and are more likely to develop it an earlier age. Leukemia and squamous cell carcinomas (SCCs) are also common tumors in cats with FIV, although the association with SCC may be confounded by outdoor exposure. Other tumors include mast cell tumors, fibrosarcomas, meningiomas, and metastatic carcinomas; some infected cats develop more than one type of neoplasia. Rarely, lymphoma may develop as a result of viral integration into the genome and disruption of proto-oncogenes.54,55 Immune dysregulation and increased circulating immune complexes in the terminal phase can lead to immune-mediated disorders, such as immune-mediated glomerulonephritis and uveitis.56 Myelodysplasia develops in some cats,57,58 which may be manifested by clinical signs of lethargy, inappetence, pallor, or evidence of bleeding tendencies such as petechial hemorrhages. The end result of infection by neurovirulent strains of FIV can be progressive behavioral changes such as increased aggression and cognitive disturbances, tremors, sleep disturbances, anisocoria, delayed reflexes, abnormal cranial nerve function, urinary and fecal incontinence, and seizures. Decreased nerve conduction velocities and abnormal electroencephalograms and brainstem evoked potentials have been documented.59 The virus does not infect neurons, but neuronal cell death occurs through multiple incompletely defined mechanisms.51 Inflammatory lesions can develop in a variety of organs in cats infected with FIV. An inflammatory myopathy has been described,60 and terminally, severe muscle atrophy can occur. Infection is associated with gut inflammation and intestinal epithelial cell damage (also known as AIDS enteropathy), which may result in chronic diarrhea and failure to gain weight.61 The extent to which chronic FIV infection contributes to inflammatory or degenerative changes in other organs, such as the myocardium and the kidneys, is not well understood, because the prevalence of cardiomyopathy and interstitial nephritis in geriatric cats not infected with FIV is high (see Table 21-3). When transplacental transmission occurs in FIV-infected queens, only some kittens in a litter may be infected. In the first year of infection, the overall rate of such transmission is around 70%.62 Rates of mother-to-kitten transmission are highest in queens that have a CD4+ count less than 200 cells/µL, those with signs of immunodeficiency, and those infected within the past 15 months.63 In utero transmission can lead to arrested fetal development, abortion, stillbirth, low birth weights, and the birth of T cell deficient kittens.62,64 Many cats infected with FIV have no signs of illness, or clinical signs that are unrelated to FIV infection. Cats in the acute phase of FIV infection may be febrile or show generalized peripheral lymphadenopathy. Behavioral abnormalities such as obtundation, aggression, and cognitive impairment may be evident in cats with terminal neurologic disease; other neurologic abnormalities include ataxia, anisocoria, and abnormal segmental reflexes. Ocular abnormalities may be present as a result of the FIV infection itself, opportunistic infections, or lymphoma, and include anterior uveitis, hyphema, pars planitis, chorioretinitis, and/or glaucoma.65 Other common physical examination findings in cats with terminal disease are periodontal disease and chronic stomatitis, signs of chronic upper respiratory tract infection, otitis externa, pyoderma, cutaneous abscesses, and a diverse and variable spectrum of disease manifestations that relate to other underlying opportunistic infections or neoplasia. For many cats, infection with FIV is diagnosed during screening efforts. Screening for infection should be performed with tests that detect antibody against FIV, because these assays have the highest overall sensitivity and are rapid and widely available (Table 21-4). It has been recommended that the retrovirus status of all cats be known regardless of the presence of absence of illness.66 Indications for testing as recommended by the American Association of Feline Practitioners (AAFP) are shown in Box 21-1. In practice, compliance with retrovirus testing is low. In a study that evaluated 967 cats with bite wounds or cutaneous abscesses that presented to veterinary practitioners from 134 practices in 30 states, the combined FeLV-FIV status of only 96 (9.9%) of the cats was known.38 In addition, despite the availability of a financial incentive for retesting, only 64 of 478 cat owners returned their cats for retesting after treatment. When positive test results occur in sick cats, the role that FIV plays as a cause of the signs may be unclear, although it is reasonable to assume that FIV may be playing a role in cats with severe stomatitis, unusual infections with intracellular pathogens such as mycobacteria, and lymphoma. With refinement and improved availability of test methodologies in the future, clinical assessment of CD4+ T cell counts, the CD4+/CD8+ ratio, and plasma viral loads may facilitate interpretation of the relationship between disease manifestations and infection and provide prognostic information, as in human patients infected with HIV.67 Common abnormalities on the CBC in cats infected with FIV include mild anemia, lymphopenia, and neutropenia. Occasionally severe anemia, thrombocytopenia, thrombocytosis, monocytopenia, or leukocytosis occur. In one large European study, neutrophil counts of FIV-infected cats were lower than those of control cats, and lymphocyte counts were higher than those of control cats.68 Leukopenia and neutropenia were more likely to be present in FIV-infected cats.
Feline Immunodeficiency Virus Infection
Etiology and Epidemiology
A
Australia, New Zealand, United States (especially western United States), South Africa, northwestern Europe, Japan, United Kingdom11–19
B
Central and eastern United States, Caribbean, central and western Europe, Brazil, eastern Japan11,14,20–25
C
United States, Canada, New Zealand, southeast Asia11,12,26–29
D
Japan, Vietnam, rarely United States21,27,31
E
Argentina10,30
F
United States10,31
Clinical Features
Physical Examination Findings
Diagnosis
Laboratory Abnormalities
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Feline Immunodeficiency Virus Infection
Only gold members can continue reading. Log In or Register to continue