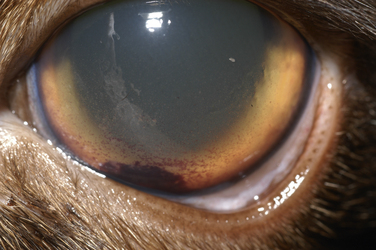
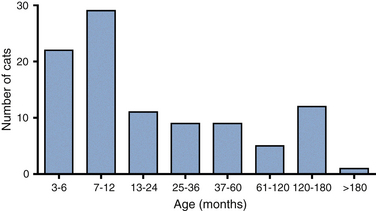
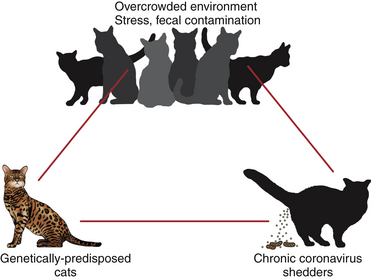
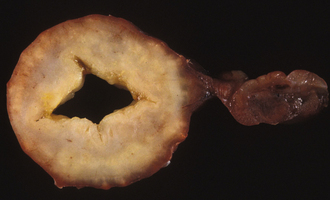
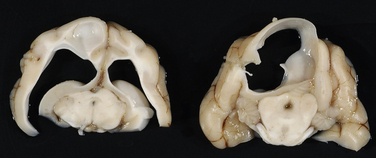
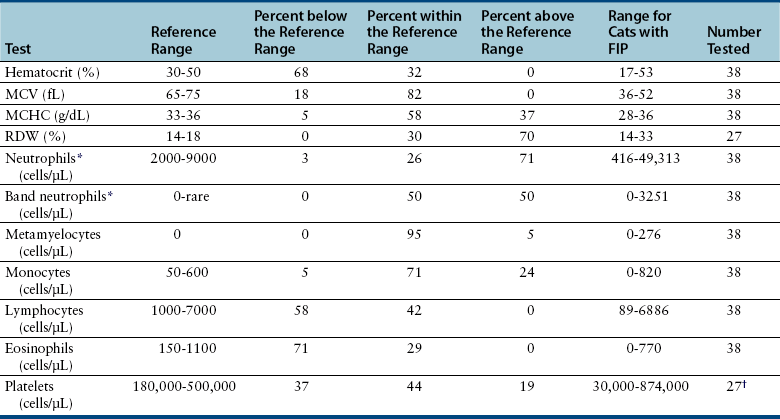
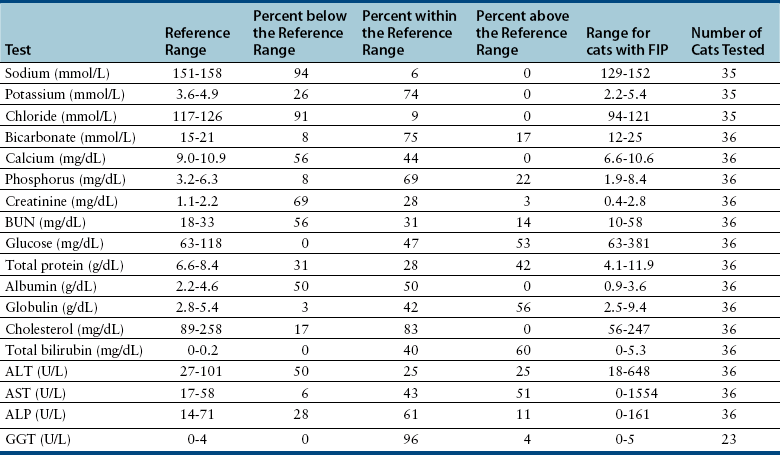
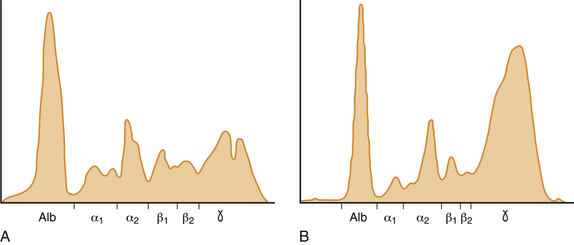
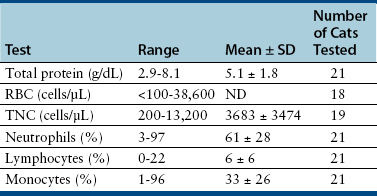
Chapter 20 Coronaviruses are large, enveloped, single-stranded RNA viruses with club-shaped spikes on their outer surface (see Figure 14-1, B). They have the largest RNA genomes of all known viruses. Feline coronaviruses (FCoV), like canine enteric coronavirus, belong to the Group 1a coronaviruses (see Box 17-1). In fact, even canine enteric coronavirus has the potential to infect cats and cause diseases similar to those caused by FCoV.2 Among FCoVs, there are two different serotypes, type I and type II, which use different receptors for cellular entry in vitro3,4 but cause the same clinical manifestations. Type I strains predominate worldwide.5–7 Type II strains, which are thought to have evolved from genetic recombination between canine enteric coronavirus and FCoV, are more readily grown in culture and so have been more extensively studied; they possess a spike protein that resembles that of canine enteric coronavirus. FCoVs cause enteric disease in cats as well as feline infectious peritonitis (FIP), a serious systemic pyogranulomatous to granulomatous disease that progresses over a period of weeks to months and, once it occurs, is ultimately always fatal. FIP is a major cause of death in young and young adult cats, especially cats from multicat environments such as purebred catteries and shelters. Wild cats, especially cheetahs, are also susceptible.8 The vast majority of domestic cats that develop FIP are 3 months to 3 years of age, with at least 50% of affected cats aged 12 months or younger (Figure 20-1). However, FIP can occur at any age, and there is a secondary peak of incidence in geriatric cats (>10 years of age), possibly as a result of suboptimal immune function. Males and sexually intact cats have been predisposed in some studies,9–11 and a disease peak may exist in the fall and winter.12 Although the disease occurs in all breeds, purebred cats are more susceptible; Abyssinians, Australian mist, Bengals, birmans, Burmese, British shorthairs, Himalayans, ragdolls, rexes, and possibly Scottish folds may be predisposed.9–14 Breed predispositions may vary geographically and temporally depending on the preferences of breeders in a region, and specific lines may be more predisposed than the breeds themselves.6 The molecular basis of genetic susceptibility to FIP is currently unclear. Siblings of cats that die of FIP may be at increased risk for FIP.15 FIGURE 20-1 Age distribution of 99 cats with necropsy-confirmed FIP at the UC Davis VMTH. An additional six cats were reported to be “juvenile” or kittens. There were 38 females (17 intact) and 66 males (22 intact). In multiple-cat household situations, cats are repeatedly infected, shed virus, and recover, but some cats remain persistently infected and chronically shed FCoV in the absence of clinical signs (Figure 20-2). More than half, and as many as 100% of cats in environments with more than six cats, become infected with FCoVs.6 The seroprevalence is lower in cats from single-cat households and among feral cats.16 However, even though the prevalence of infection in multicat households is high, fewer than 10% of cats from large, multicat households ultimately develop FIP. Thus, although the incidence of infection is high, the incidence of disease in single- or two-cat households is only around 1 in 5000; in catteries it is around 5% to 10%.17,18 Provided they are unrelated by birth, cats in households with a history of FIP are not more likely to develop FIP than cats in households without FIP.19 Thus, FIP is usually a sporadic disease that does not spread from one cat to another. However, every few years, epidemics of disease can occur in catteries or shelters, with mortality rates that exceed 10%.12 Because it is an enveloped virus, FCoV is readily inactivated by disinfectants and generally survives less than a day or two at room temperature. However, the possibility of prolonged survival (up to 7 weeks) in the environment under certain conditions has been suggested.15,20 In this situation, fomites might play an important role in transmission. FIGURE 20-2 Interplay between genetics, virus shedding, and environment in feline coronavirus infections and FIP. The epidemiology and pathogenesis of FIP has both fascinated and confused veterinary virologists worldwide for decades. The most widely accepted theory (the “internal mutation hypothesis”) is that cats are initially infected with a low-pathogenicity coronavirus after oronasal exposure, which results either in no signs, or mild enteric disease. This low-pathogenicity virus has been referred to as feline enteric coronavirus in some publications in order to distinguish it from virulent FIP virus. The use of this name has been controversial, because although the virus is primarily confined to the gastrointestinal tract (and especially colonic epithelial cells), FCoV RNA can also be found in blood and tissue macrophages of cats that do not have FIP.21,22 In some infected cats, the low-pathogenicity virus is believed to mutate to a virulent strain that can multiply within macrophages without hindrance by the immune system and incite a systemic pyogranulomatous vasculitis. The mutation may occur shortly after initial infection, or years later, which may explain why some indoor cats from single-cat households develop FIP several years after they are acquired. Virulent strains may not be able to replicate effectively within the gut,23 which may be the reason why cat-to-cat transmission of FIP does not occur, yet the disease can be transmitted effectively by inoculating naïve cats with effusion from a cat with FIP. Factors that contribute to immunosuppression, such as concurrent viral infection, stress due to overcrowding, surgery, or transport, and especially genetic factors may allow viral replication and mutation to proceed unchecked. Simultaneous immune compromise of a large number of cats, such as in a shelter situation, may explain epidemics of FIP. Other risk factors for FIP include regular introduction of new cats to a cattery and the proportion of cats in a cattery that shed coronavirus chronically.12 There is no distinct mutation that allows avirulent FCoV strains to be differentiated from virulent strains, and therefore no diagnostic test exists that distinguishes FIP from benign FCoV strains. However, mutations in the spike protein gene,24,25 membrane protein gene,26 and the nonstructural 3c and 7b genes23,27–29 may play a role. In particular, the 3c gene appears to be disrupted in many (but not all) virulent FCoV strains. The other hypothesis proposed to explain the pathogenesis of FIP is that distinct circulating virulent and avirulent FCoV strains exist, and the combination of infection with a virulent FCoV and an individual cat’s genetic and environmental predispositions leads to FIP.25 It has also been suggested that both hypotheses may play a role.30 Cats are usually infected with FCoV by oronasal exposure to virus in feces or fomites contaminated with fecal material. Shared litter boxes are thought to play a major role in transmission.15 Replication of low-pathogenicity strains of FCoV in epithelial cells at the tips of intestinal villi may be associated with no signs, or acute or chronic, persistent or intermittent small-bowel diarrhea, and less commonly, vomiting and/or inappetence. Transient upper respiratory signs have been reported in some cats on initial infection with FCoV.15 Virus is shed in the feces from 1 week after infection. Some cats then shed large quantities of virus continuously for life.12,22,31–33 Both serotype I and serotype II strains appear to enter macrophages via a lectin receptor known as fDC-SIGN (feline dendritic cell-specific intercellular adhesion molecule grabbing non-integrin receptor).3,34 Replication of virulent FCoV strains within macrophages results in two forms of disease, which reflect the immune response mounted by the host. FIP is an immune complex disease. Noneffusive (“dry”) FIP occurs in cats that mount a partial CMI response and is characterized by pyogranulomatous to granulomatous inflammation within a variety of organs, but especially the mesenteric lymph nodes, kidneys, liver, lungs, brain, and eye. Solitary or multifocal granulomas of the intestinal wall also occasionally develop, especially in the region of the ileocecal junction (Figure 20-3).15 Effusive (“wet”) FIP occurs in cats that are unable to mount an immune response and is characterized by accumulation of high protein exudates in the thorax and/or abdomen, which typically contain low numbers of cells. Production of vascular endothelial growth factor by infected monocytes may be lead to increased vascular permeability and contribute to cavitary effusion.35 Many cats have a mixture of both forms of the disease, and noneffusive disease may progress to effusive disease. Infection itself results in immune dysregulation, with a profound, virus-induced depletion of CD4+ and CD8+ cells; production of TNF-α, granulocyte-macrophage colony stimulating factor (GM-CSF), and granulocyte colony-stimulating factor (G-CSF) by infected macrophages; impaired IFN-γ production; and hypergammaglobulinemia.36–38 The mechanism of T-cell depletion is not clear, as the virus does not infect lymphocytes, only monocytes and macrophages. Infection of antigen-presenting cells, specifically dendritic cells, has been hypothesized to lead to T-cell apoptosis. Progressive immune system failure may be associated with a conversion to predominantly effusive disease manifestations. Despite the profound T-cell deficiency that accompanies FIP, opportunistic infections are rarely reported. Nevertheless, concurrent infections with retroviruses and Toxoplasma gondii and opportunistic bacterial infections can occur;6 the author is aware of one cat that was co-infected with Sporothrix schenckii. FIGURE 20-3 Colonic mass removed at surgery from a 7-month-old female spayed domestic shorthair with anorexia and hematochezia. Histopathology showed severe, multifocal coalescing pyogranulomatous colitis and lymphadenitis. (Courtesy of the University of California, Davis Veterinary Anatomic Pathology service.) The incubation period for FIP is highly variable. Kittens usually become infected at 4 to 8 weeks of age, when maternal antibody begins to wane, but infections have been reported in kittens as young as 2 weeks of age.20 Disease may occur a few weeks after infection or years later, but most often it occurs 6 to 18 months after initial infection.19 Even after the onset of systemic pyogranulomatous inflammatory disease, clinical signs may not be apparent for months. In support of this, lesions consistent with FIP have been found incidentally in cats during abdominal surgery such as ovariohysterectomy.20 The clinical signs of FIP often change over time and depend on the organs affected and the relative predominance of inflammatory versus effusive disease manifestations. The most common signs are lethargy and inappetence, as well as a fluctuating fever that does not respond to antibacterial drug treatment. Nevertheless, many cats are bright, appetent, and in good body condition early in the course of illness. Some cats have increased thirst and urination, possibly secondary to pyrexia. Ultimately, weight loss develops, but owners of cats that develop abdominal distention may mistake the distention for weight gain or pregnancy. Stunted growth may occur in affected kittens. Pleural effusion may be associated with tachypnea and respiratory distress. Testicular enlargement may occur in cats with serositis that involves the tunica vaginalis. FIP is responsible for approximately 10% of pericardial effusions in cats, the third most common cause of pericardial effusion after cardiomyopathy and neoplasia.39 Rarely, pericardial effusion results in cardiac tamponade. Pyogranulomatous or granulomatous inflammation may lead to mesenteric lymphadenomegaly, irregular renomegaly, intestinal masses, hepatomegaly, icterus, pneumonia, uveitis, chorioretinitis, and, rarely, nodular skin lesions. Neurologic signs, which can include focal or generalized seizures, occur in at least 10% of cats with FIP and result primarily from meningoencephalitis, meningomyelitis, ependymitis, choroiditis, and obstructive hydrocephalus. Obstructive hydrocephalus occurs secondary to choroiditis and ependymitis (Figure 20-4). In one study, FIP was responsible for almost half of all neurologic disease in 97 cats due to infectious or inflammatory causes.40 Occasionally profound anemia occurs secondary to immune-mediated hemolysis13,14 or possibly microangiopathic damage, whereby erythrocytes are lysed as they travel through inflamed blood vessels. Immune-mediated glomerulonephritis has also been reported, and FIP should always be considered in cats with protein-losing nephropathy, which is otherwise rare in cats.41 Uncommonly, lameness occurs as a result of synovitis.6 FIGURE 20-4 Obstructive hydrocephalus in an 8-month-old male neutered exotic shorthair cat that developed ataxia and head tremors. Hydrocephalus and secondary cerebellar herniation were found at necropsy. Histopathology revealed severe, multifocal pyogranulomatous meningoencephalitis, choroiditis, and ventriculitis, and pyogranulomatous inflammatory lesions were also found throughout the thoracic and abdominal viscera. (Courtesy of the University of California, Davis Veterinary Anatomic Pathology service.) Physical examination findings in cats with FIP reflect the type of disease present (effusive versus noneffusive) and the location where lesions occur. Cats with respiratory tract involvement may show tachypnea, and if there is pleural effusion, a rapid, shallow breathing pattern and muffled heart and lung sounds may be present. Other signs include pyrexia, dehydration, mucosal pallor or icterus, a thin body condition, and evidence of ascites. Abdominal palpation may reveal hepatomegaly, irregular renomegaly, and/or abdominal mass lesions that result from mesenteric lymphadenomegaly or intestinal pyogranulomas. Sometimes pain is appreciated on abdominal palpation, which may reflect pancreatic involvement in some cats. Testicular enlargement may be detected in intact male cats. A wide range of neurologic signs may be present, such as obtundation, twitching, tremors, behavioral changes, nystagmus, hyperesthesia, exaggerated segmental reflexes, ataxia, urinary incontinence, or cranial nerve defects. Ocular signs include conjunctivitis, mucopurulent ocular discharge, thickening and hyperemia of the nictitans, uveitis with dyscoria or anisocoria, aqueous flare, keratic precipitates, hypopyon, hyphema, chorioretinitis, perivascular infiltrates, retinal detachment, or blindness (Figure 20-5). A mild, nonregenerative anemia is often present in cats with FIP, and sometimes severe anemia occurs, which is usually poorly regenerative or nonregenerative (Table 20-1). Microcytosis may be present. Examination of erythrocyte morphology occasionally reveals schistocytosis, mild normoblastosis, or agglutination. There may be a leukocytosis due to a neutrophilia and monocytosis, or leukopenia. Lymphopenia occurs in more than 50% of affected cats, and eosinopenia is also common. In some cats, a left shift and evidence of toxic neutrophils are seen. Mild to moderate thrombocytopenia is common in cats with noneffusive disease and may reflect the presence of disseminated intravascular coagulation or immune-mediated platelet destruction. However, thrombocytosis can also occur. TABLE 20-1 Complete Blood Count Findings at Admission in 38 Cats with Necropsy-Confirmed Feline Infectious Peritonitis at the UC Davis VMTH FIP, Feline infectious peritonitis; RDW, red cell distribution width. ∗22 (58%) had evidence of toxic neutrophils. †A smear was evaluated manually for 37 of the 38 cats. The presence of macroplatelets were reported for 18 (49%) of cats. Many cats with FIP have hyperproteinemia due to hyperglobulinemia, which results from a polyclonal gammopathy (Figure 20-6). Rarely, a monoclonal gammopathy can occur.42 Total protein concentrations may be as high as 12 g/dL (Table 20-2).20 In one study, hyperglobulinemia was present in 50% of cats with effusion and 70% of cats without effusion.43 Globulin concentration may decrease terminally, so cats with advanced disease may have protein concentrations that are within the reference range.14 Hypoalbuminemia is often present because of liver involvement, leakage from damaged vessels, urinary loss in cats with glomerulonephritis, or inflammation (albumin is a negative acute-phase reactant protein). Thus, the serum albumin:globulin ratio may be more useful than the globulin alone for diagnosis; ratios less than 0.8 are uncommon (but not impossible) in cats with FIP, so they help to rule out (but not to rule in) a diagnosis of FIP.44,45 Other variable findings include hyponatremia, hypokalemia, hypochloremia, hyperglycemia, azotemia, increased liver enzyme activities, hypocholesterolemia, and hyperbilirubinemia. The cause of hyperbilirubinemia is not clear, but it may result from hemolysis, hepatic necrosis, and/or cholestasis. TABLE 20-2 Findings on Serum Biochemistry Analysis in 36 Cats with Necropsy-Confirmed Feline Infectious Peritonitis at the UC Davis VMTH FIGURE 20-6 A, Densitometric scan of serum protein electrophoresis of normal feline serum. B, Scan from a 9-month-old male neutered domestic shorthair cat with FIP. There is a polyclonal gammopathy, represented by a broad peak in the γ-globulin region, with a mild decrease in the albumin and mild increases in the α2 and β1 fractions. (A redrawn from Baker RJ, Valli VE. Electrophoretic and immunoelectrophoretic analysis of feline serum proteins. Am J Vet Res 1988;52[3]:308-304.) Measurement of α1-acid glycoprotein (an acute phase protein) has been suggested for diagnosis, because serum concentrations often exceed 1500 µg/mL in cats with FIP.20,46,47 However, α1-acid glycoprotein concentrations also increase with other inflammatory diseases.20 In addition to thrombocytopenia, abnormalities of coagulation in cats with FIP include prolonged prothrombin time and partial thromboplastin time as a result of severe liver injury, and increased fibrin degradation product or D-dimer concentrations.15 The “classic” FIP effusion fluid is a high-protein (greater than 3.5 g/dL) exudate that contains a low number of nucleated cells (<5000 cells/µL), usually nondegenerate to mildly degenerate neutrophils and macrophages (Table 20-3). Erythrophagocytosis, leukophagia, and reactive mesothelial cells can be observed in the fluid from some cats. Grossly, the fluid has a yellow appearance and may contain fibrin clots. However, the total protein content and cell counts of abdominal and pleural effusions vary considerably, which complicates the diagnosis for some cats with effusive disease. Very rarely, chylous effusions occur.48 An effusion albumin/globulin ratio below 0.4 is suggestive of FIP.49 TABLE 20-3 Composition of Body Cavity Effusions from 21 Cats with Necropsy-Confirmed Feline Infectious Peritonitis at the UC Davis VMTH Nineteen specimens were abdominal and two were pleural effusions. ND, Not determined; SD, standard deviation; TNC, total neutrophil count.
Feline Coronavirus Infection
Etiology and Epidemiology
Clinical Features
Physical Examination Findings
Diagnosis
Laboratory Abnormalities
Serum Biochemical Tests
Coagulation Profile
Analysis of Effusion Fluid
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Feline Coronavirus Infection
Only gold members can continue reading. Log In or Register to continue