Chapter 270 Bartonella spp. are fastidious, vector-transmitted, gram-negative bacteria in the α-2 Proteobacteria family Bartonellaceae. At least 24 named Bartonella spp. are highly adapted to and likely evolved with mammalian reservoir hosts, in which long-term, apparently asymptomatic bacteremia occurs (Breitschwerdt et al, 2010; Guptill, 2010a). Since the first report of feline B. henselae infection in 1992, natural infection of cats with five Bartonella spp. (B. henselae, B. clarridgeiae, B. koehlerae, B. quintana, and B. bovis [formerly B. weissii]) has been reported. Feline infections with species other than B. henselae or B. clarridgeiae are uncommon (Chomel and Kasten, 2010). Approximately 14 Bartonella spp. are considered zoonotic; all five of the Bartonella spp. identified in cats are zoonotic. The primary zoonotic Bartonella sp. associated with cats is B. henselae, which causes long-term bacteremia in healthy cats. It has been detected by polymerase chain reaction (PCR) in tissues of numerous other mammalian species, some of which are dogs, seals, whales, horses, and wild felids (Breitschwerdt et al, 2010). Domestic cats are considered the primary mammalian reservoir and vector for human infections with B. henselae. B. henselae causes or has been associated with cat-scratch disease (CSD), bacillary angiomatosis, bacillary peliosis, relapsing fever with bacteremia, meningitis, encephalitis, neuroretinitis, endocarditis, and many additional conditions in humans (Chomel and Kasten, 2010). B. clarridgeiae was associated serologically with a CSD-like condition in two people (Breitschwerdt et al, 2010). B. koehlerae, isolated from several healthy cats, has been associated with human and canine endocarditis (Ohad et al, 2010) but did not cause clinical signs in cats inoculated experimentally. Although B. quintana has been identified in cats, it has not been established that it is transmitted zoonotically by cats. Humans are the reservoir host for B. quintana, which caused trench fever in World War I and also causes endocarditis, bacillary angiomatosis, bacillary peliosis, and chronic lymphadenomegaly in people (Chomel et al, 2006; Kaiser et al, 2011). This chapter focuses on B. henselae, the feline-associated Bartonella spp. for which the most information is known. Exposure of cats to Bartonella spp. is common worldwide. B. henselae bacteremia occurs in approximately 5% to 40% of cats in the United States on average, with higher prevalence in warmer, more humid regions with high flea prevalence. In some cat colonies, Bartonella seroprevalence reaches 90% or more. Most cats with Bartonella bacteremia are infected with B. henselae; between 10% and 31% of cats surveyed with Bartonella bacteremia were infected with B. clarridgeiae. B. koehlerae and B. bovis were detected in only a few cats. Bartonella spp. also were identified in wild felids in North America, Brazil, and Africa (Chomel et al, 2009b; Guptill, 2010a). Coinfection of cats with multiple B. henselae genetic types and with B. henselae and B. clarridgeiae is reported. There are regional differences in prevalence of infection of cats with different types of B. henselae, and there is remarkable molecular diversity among B. henselae isolates (Chomel et al, 2009b). Genomic variation in B. henselae during the course of infection in cats likely enhances the ability of B. henselae to persist in cats for prolonged periods. Genetics also may be a factor in the pathogenicity of various Bartonella spp. isolates. Bartonella henselae is transmitted naturally among cats by cat fleas (Ctenocephalides felis felis). The primary mode of transmission is via intradermal exposure to flea feces; it does not appear that transmission occurs via flea saliva. Ticks may have a role in transmission, and Bartonella spp. also have been detected in biting flies, lice, and other arthropods (Tsai et al, 2011). Transmission among cats does not appear to occur normally transplacentally or directly through grooming or sharing of food dishes and litter boxes when fleas are absent. Feline bacteremia with B. henselae is chronic and recurrent. Experimentally infected cats had relapses of bacteremia occurring at irregular intervals of between 1 and Cats have robust cellular and humoral immune responses to Bartonella spp. infections, and immunosuppression by Bartonella spp. infection has not been documented. Cell-mediated immunity appears important in reducing the level of bacteremia in experimentally infected cats. Cats maintained normal CD4 and CD8 lymphocyte numbers and ratios in one experimental study, whereas in another study some experimentally infected cats had transiently decreased CD4 lymphocyte numbers (Guptill, 2010a). Prior infection with Bartonella spp. does not appear to confer broad-based immunity to reinfection; most cats are susceptible to infection with Bartonella strains and species to which they have not been exposed previously (Breitschwerdt et al, 2010; Guptill, 2010a). Bartonella spp. are generally intracellular bacteria, and B. henselae has been detected within erythrocytes of naturally infected cats. Bartonella spp. also may be located intracellularly in vascular endothelial cells of infected cats as has been suggested for rats infected with B. tribocorum. Extracellular B. henselae also are detected in blood and other tissues of infected cats (Chomel et al, 2009a; Guptill, 2010). Most cats experimentally infected with Bartonella spp. exhibited no clinical signs. Clinical signs that did occur were, with some notable exceptions, generally mild, and varied with the strain of B. henselae used for inoculation (Guptill, 2010b). Cats developed induration or abscess at intradermal inoculation sites between approximately 2 and 21 days after inoculation. Other transient clinical findings included generalized or localized peripheral lymphadenomegaly that persisted for approximately 6 weeks after inoculation. Fever (>103° F; 39.4° C) occurred during the first 48 to 96 hours after inoculation and again for 24 to 48 hours at approximately 2 weeks after inoculation. Some cats were lethargic and anorexic when febrile. Mild neurologic signs (e.g., nystagmus, transient whole body tremors, decreased or exaggerated responses to external stimuli, mild behavior changes), and epaxial muscle pain also were reported in some cats. In one study, three of six cats infected by exposure to C. felis developed fever and one cat was euthanized because of severe cardiac disease. Reproductive failure occurred in some cats. There were no reported clinical signs in cats experimentally infected with B. koehlerae or the proposed species B. rochalimae (Breitschwerdt et al, 2010; Guptill, 2010a; Chomel and Kasten, 2010). Complete blood counts, serum biochemical tests, and urine analysis were normal in most experimentally infected cats. A few cats had transient mild anemia early in the course of infection, and some had persistent eosinophilia. Mature neutrophilia occurred in some cats during periods of skin inflammation. Cats had hyperplasia of lymphoid organs, small foci of lymphocytic, pyogranulomatous, or neutrophilic inflammation in multiple tissues (lung, liver, spleen, kidney, heart), and small foci of necrosis in the liver or spleen (Breitschwerdt et al, 2010; Guptill, 2010a). Clinical signs are not reported commonly in naturally infected cats. There were no clinical signs reported in 65 naturally infected cats in one study. Bartonella infection has been associated with uveitis in some cats (Breitschwerdt et al, 2010; Guptill, 2010b). Bartonella spp. were associated with endocarditis in two naturally infected cats (Chomel et al, 2009). Whether members of the genus Bartonella contribute to previously described instances of argyrophilic bacteria in lymph nodes of cats with persistent lymphadenomegaly is unknown. Bartonella DNA was not found in tissues of 14 cats with plasmacytic pododermatitis, or 26 cats with peliosis hepatis, and immunohistochemical staining was negative for Bartonella spp. in these cats (Guptill, 2010). A potential causative role of Bartonella spp. in chronic diseases of cats has been proposed because Bartonella bacteremia in cats often is prolonged, and systemic inflammation is documented in infected cats. Contribution of Bartonella infections to development of chronic illnesses of cats has not been verified. Associations have been reported between seropositive status and gingivitis, stomatitis, lymphadenomegaly, unspecified urinary tract disorders, and hyperglobulinemia (Breitschwerdt et al, 2010; Chomel et al, 2006; Guptill, 2010b). However, the use of serology for establishing Bartonella spp. infection appears to be limited, and conclusions drawn from studies that rely solely on serologic methods for diagnosis of Bartonella spp. infection should be interpreted with caution. Clinical conditions proposed as attributable to feline bartonellosis also may result from other causes, and determining which cats have clinical signs attributable to Bartonella spp. infection is difficult. Case-control studies evaluating naturally infected cats have failed to prove association of Bartonella spp. with anemia, gingivostomatitis, neurologic conditions, high serum trypsin-like immunoreactivity, inflammatory polyps, chronic rhinosinusitis, or uveitis (Guptill, 2010b; Stiles, 2011). In some of these studies, animals seropositive for Bartonella spp. were less likely to be affected by the clinical condition studied than were serologically negative animals. The prevalence of Bartonella DNA in the blood of febrile cats was almost statistically significantly greater (P = 0.057) than the prevalence of Bartonella DNA in the blood of afebrile cats (Breitschwerdt et al, 2010; Guptill, 2010b).
Feline Bartonellosis
Epidemiology and Pathogenesis
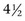 months. Persistent natural relapsing bacteremia in cats because of reinfection of cats with different strains of B. henselae over time also is reported (Breitschwerdt et al, 2010; Guptill, 2010a).
months. Persistent natural relapsing bacteremia in cats because of reinfection of cats with different strains of B. henselae over time also is reported (Breitschwerdt et al, 2010; Guptill, 2010a).
Clinical Findings
Experimental Studies
Natural Infection
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


