Chapter 22 Exotic Pets∗
∗ Reprinted with permission from the following:
General Introduction and Required Equipment
Endoscopy has proven to be a most useful diagnostic tool in veterinary medicine. In the field of zoological medicine, the application of diagnostic endoscopy has shown great promise in a variety of species but has probably been most exploited by avian veterinarians.1,2 Great strides have been made over the past decade in many areas of zoological endoscopy; however, because of space limitations in this chapter, only a handful of select procedures have been described for those species likely to be encountered in companion animal practice, namely, exotic mammals (rabbits, ferrets, and rodents), companion and working birds (psittacines and raptors), companion reptiles (lizards, snakes, turtles, and tortoises), and ornamental fish. Most of the modern developments and advances have not been included, and readers are directed to the references at the end of each section for more complete details.
Instrumentation
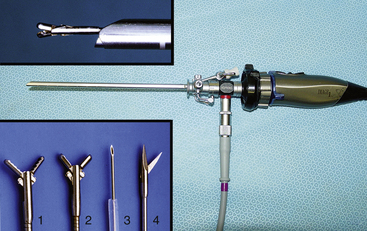
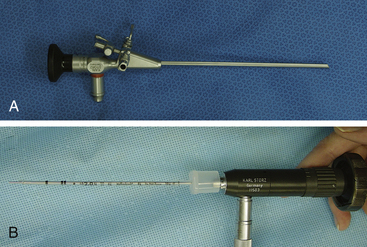
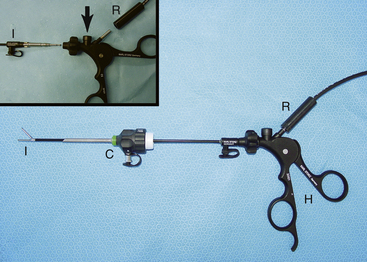
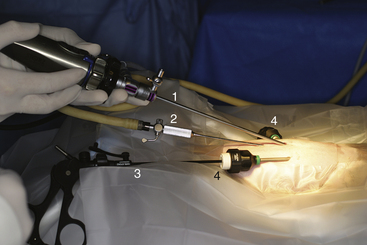
Given the variation in size and the nature of the species and procedures that may be performed, a variety of different scopes and instruments are often required (Table 22-1).3–5 For most practices the 2.7-mm telescope and 4.8-mm operating sheath offers the greatest versatility, which can be built on as individual practice caseload dictates. This system offers several advantages, including single-entry procedures, ports for insufflation or saline infusion, and an operating channel for the introduction of 1.7-mm instruments (Figure 22-1). In addition, the 1.9-mm telescope with integrated sheath and the 1-mm semirigid miniscope are extremely useful for smaller species (Figure 22-2). For multiple-entry endoscopy, the recent application of human pediatric 2- and 3-mm instruments to exotic species has enabled coelioscopy, laparoscopy, and thoracoscopy to become a reality (Figures 22-3 and 22-4).6
Table 22-1 Endoscopic Instrumentation for Companion Exotic Mammals, Reptiles, Birds, and Fish
| Equipment description | Primary indications |
|---|---|
| Visualization and documentation | |
| Required for all endoscopy procedures | |
| Endoscopes | |
| 1 mm × 20 cm semirigid miniscope, 0 degree | Stomatoscopy, otoscopy, rhinoscopy, tracheoscopy in animals less than 1 kg |
| 1.9 mm × 18.5 cm telescope, 30-degree oblique, with integrated 3.3-mm operating sheath | Stomatoscopy, otoscopy, rhinoscopy, tracheoscopy, gastroscopy, colonoscopy, vaginoscopy, cloacoscopy, laparoscopy/coelioscopy, and thoracoscopy in animals between 500 g and 3 kg |
| Stomatoscopy, otoscopy, rhinoscopy, tracheoscopy, gastroscopy, colonoscopy, vaginoscopy, cloacoscopy, laparoscopy/coelioscopy, and thoracoscopy in animals between 1 kg and 8 kg | |
| 5 mm × 8.5 cm otoendoscope, 0 degree, with integrated operating sheath | Stomatoscopy and otoscopy in animals between 3 kg and 50 kg |
| Flexible instruments for use with operating sheaths | |
| For use with 1.9-mm telescope and integrated sheath | |
| For use with 2.7-mm telescope, 4.8-mm operating sheath, and 5-mm otoendoscope | |
| Insufflation | |
| CO2 insufflator with silicone tubing | Used for insufflation during laparoscopy |
| Sterile saline suspended above endoscopy table with intravenous drip line to a port on the operating sheath | Used for sterile saline infusion for otoscopy, rhinoscopy, cystoscopy, vaginoscopy, colonoscopy, cloacoscopy, and coelioscopy |
| Rigid instruments and cannulae for multiple-entry laparoscopy and thoracoscopy | |
| Used with the 1.9-mm telescope for laparoscopy, thoracoscopy, and coelioscopy in animals less than 500 g | |
| Used with the 2.7-mm telescope for laparoscopy, thoracoscopy, and coelioscopy in animals less than 8 kg to 10 kg | |
| Radiosurgery equipment | |
3.8- or 4.0-MHz dual radiofrequency unit (for example, Surgitron, Ellman International Inc.) with foot pedal | Enables endoscopic instruments to be used as monopolar devices and facilitates bipolar coagulation |
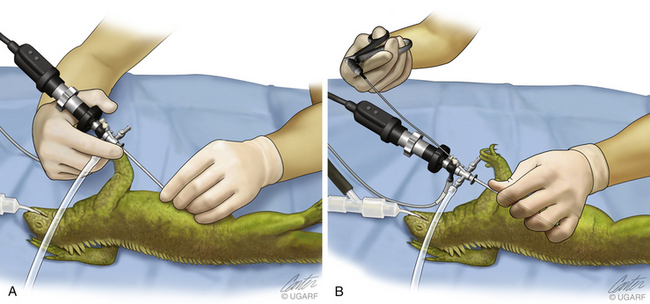
The fact that most exotic pets weigh less than 2 kg requires careful control of the endoscope; the eyepiece and camera should be supported with the superior hand, and the terminal shaft should be held by the thumb and forefinger of the inferior hand (Figure 22-5, A). Handling the endoscope in this fashion provides fine control without tremor. Biopsy specimens can be easily harvested from most structures. However, when an instrument is inserted and manipulated, it is necessary to change from a two-handed hold to a one-handed technique. The usual thumb and forefinger support of the endoscope shaft by the inferior hand is now adjusted so that the left hand can take the entire weight of the sheath–scope–camera system. The thumb is slid up the shaft of the sheath, and the fingers are curled over the top to encircle the sheath. Now that the sheath is grasped in a fist with the sheath further supported by the thumb to prevent rotation, the right hand can be removed to pick up and insert an instrument down the operating channel (see Figure 22-5, B). This can only be performed using a correctly sheathed telescope; otherwise, damage will occur.
The 1.0- and 1.7-mm grasping forceps are useful for manipulation of tissues, débridement, and retrieval of foreign objects, including parasites. The fine aspiration/injection needle can be used for the aspiration of fluid from cystic structures where biopsy may be contraindicated because of postsampling leakage. The remote injection needle can be used for remote aspiration, irrigation, and drug administration. The 1.0- and 1.7-mm flexible biopsy forceps are used to harvest tissue samples for histopathologic and microbiologic analysis in patients as small as 30 g. The small sample size usually permits the collection of several biopsy samples for multiple laboratory tests and the use of serial sampling to monitor disease progression over time. To take a tissue sample, the biopsy forceps are inserted down the operating channel and into the field of view. It is much easier to advance and manipulate the sheath–scope–instrument as a single device than to try to keep the sheath–scope still and independently move the biopsy forceps back and forth. With the biopsy forceps held open, the sheath–scope–instrument is advanced to the tissue of interest and when tissue enters the biopsy cup, the handle is released. These instruments are delicate, and the biopsy handle is only required to open the biopsy jaws. The handle’s spring mechanism is usually sufficient to take a soft tissue biopsy without additional manual pressure, as long as the instrument is sharp. Clamping down on the handle will damage the forceps and increase biopsy crush artifact. Some organs may be protected by a more fibrous membrane. The fixed blade of the scissors is inserted at a shallow angle through the membrane, and the sheath–scope–scissors are advanced as a single unit, cutting the membrane as they proceed. The scissors can then be replaced by the biopsy forceps to take a sample through the capsular incision.
A definitive diagnosis is essential for maximizing treatment success, and many exotic animal cases are unsuccessfully managed simply because of a failure to identify the problem. Definitive diagnosis relies on the demonstration of a host pathologic response (as determined by paired rising antibody titers, histopathologic or, less reliably, cytologic analysis) and the causative agent (as determined by microbiologic, parasitologic, or toxicologic analysis). There are few reliable serologic tests available for most exotic species, and those that are available require a minimum of 2 to 3 weeks between samples. It is therefore clear that tissue samples are required to make a speedy diagnosis, and short of necropsy, endoscopy offers the least invasive method to collect such material. Consequently, clinicians will discover that diagnostic endoscopy offers an unparalleled ability to diagnose disease and maximize treatment success in these demanding patients.
1. Brearley M.J., Cooper J.E., Sullivan M. Endoscopy in exotic species. In Color atlas of small animal endoscopy. St Louis: Mosby-Year Book; 1991.
2. Burrows C.F., Heard D.J. Endoscopy in nondomestic species. In Tams T.R., editor: Small animal endoscopy, ed 2, St Louis: Mosby, 1999.
3. Hernandez-Divers SJ: Small mammal endoscopy. In Quesenberry KE, Carpenter JW, editors: Ferrets, rabbits and rodents: clinical medicine and surgery, ed 3, Philadelphia, Saunders (in press).
4. Hernandez-Divers S.J. Diagnostic and surgical endoscopy. In Raiti P., Girling S., editors: Manual of reptiles, ed 2, Cheltenham, England: British Small Animal Veterinary Association, 2004.
5. Hernandez-Divers S.J., Hernandez-Divers S.M. Avian diagnostic endoscopy. Compend Cont Educ Vet. 2004;26:839–852.
6. Hernandez-Divers S.J. Minimally-invasive endoscopic surgery of birds. J Avian Med Surg19. 2005:107-120.
Selected Endoscopy Procedures for Exotic Mammals (Ferrets, Rabbits, and Rodents)
With more than 10 million pet rabbits (Oryctolagus cuniculus), ferrets (Mustela putorius furo), and rodents (order Rodentia) in the United States, these exotic mammals represent the third largest group of companion mammals (behind dogs and cats).1 The majority of rabbits, ferrets, and rodents presented to practitioners are less than 2 kg, and given their small size, they are ideal candidates for minimally invasive diagnostic and surgical endoscopy. Indeed, in some situations the development of endoscopy has enabled many procedures to be performed for the first time or with significantly reduced morbidity and mortality rates compared with traditional surgery (e.g., rhinoscopy versus rhinotomy). Considerable advances in exotic animal endoscopy have been made over the past 5 years, and further development and refinement seem assured.2 However, because of space restrictions, only stomatoscopy, rhinoscopy, and laparoscopy can be briefly covered here. For otoscopy, vaginoscopy, tracheobronchoscopy, cystoscopy, gastrointestinal endoscopy, and thoracoscopy, readers are directed to a complete review on the subject.3
Instrumentation
Given the variation in size and the nature of the procedures that may be performed, a variety of different scopes and instruments are available (see Figure 22-1). For most practices the 2.7-mm system offers the greatest versatility and can be built on as individual practice caseload dictates. This system offers several advantages, including single-entry procedures, ports for air or saline infusion, and an operating channel for the introduction of 1.7-mm instruments (see Figure 22-1). In addition, the 1.9-mm telescope with integrated sheath and the 1-mm semirigid miniscope are extremely useful for smaller mammals (see Figure 22-2). For multiple-entry endoscopy, the recent application of human pediatric 2- and 3-mm instruments to exotic animal endoscopy has enabled laparoscopy and thoracoscopy to become a reality (see Figures 22-3 and 22-4).4
Procedures
In general, the approach to exotic mammal endoscopy is similar to that used in domesticated dogs and cats, and much can be learned and applied from the domestic animal and human literature.5,6 However, in addition to the anatomic peculiarities, the exotic mammal endoscopist must be more precise, given the confines within these smaller species. It is therefore particularly important to use the finger and the thumb of the inferior hand to support the tip of the telescope to ensure accurate control at all times.
Stomatoscopy
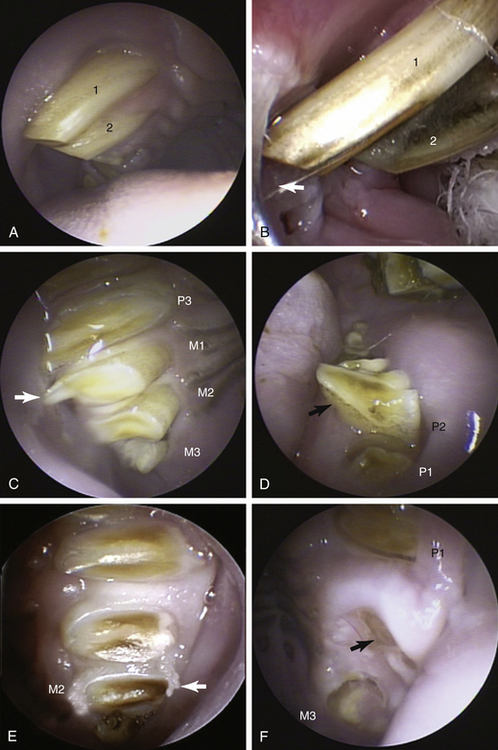
Dental disease is undoubtedly one of the most common presentations for rabbits and rodents, and stomatoscopy under general anesthesia ensures a far more detailed examination than can be achieved in the conscious animal in the examination room.2,7,8 The limited access to the oral cavity may preclude the use of endotracheal tubes; however, anesthetic gas and oxygen can be supplied via nasal intubation or by placing a small face mask over the nostrils. It is important to give consideration to the use of active scavenging from the area to avoid anesthetic gas exposure to staff. Alternatively, injectable anesthetic agents may be used, and although this does not negate the need for supplying oxygen via a nasal line or mask, it does reduce staff exposure to inhalant agents. The animal is positioned in sternal recumbency with the head supported and mouth held open using a “rodent/rabbit table retractor restrainer” (Sontec Instruments Inc., Centennial, Colo.), and “cheek spreaders” (Sontec Instruments Inc.). The 1.9- and 2.7-mm telescopes are preferred because the 30-degree angle provides a better view of the occlusal surfaces: with the light guide cable down, the view is angled toward the maxillary arcades, and with the light guide cable up, the mandibular teeth are seen preferentially. The lingual, buccal, and occlusal aspects of every tooth should be evaluated with the use of appropriately sized and curved dental probes. Tooth laxity, exudates, and gingival changes should be noted. In the vast majority of the small herbivores, the most commonly encountered malocclusions involve overgrowth to the lingual aspect of the lower arcades and buccal aspect of the upper arcades (Figure 22-6). Once identified the malocclusion should be trimmed with either ronguers or, preferably, a motorized dental handpiece that is less likely to result in dental fracture. During dental trimming it is vital that the telescope is either protected with a guard or temporarily removed and then reinserted to evaluate the teeth after reduction of the malocclusion. The telescope can also be used periodically to evaluate the intraoperative progress of premolar or molar extractions or to examine the cavity left after extraction. Indeed, the telescope has also been used to target flushing and antimicrobials into dental cavities via the oral cavity. These techniques have been used to successfully treat retrobulbar abscesses in rabbits via the oral cavity, thereby avoiding enucleation.9 Although rare, soft tissue masses may be sampled with the use of the 1.7-mm biopsy forceps, and foreign bodies may be removed with retrieval forceps.
Rhinoscopy
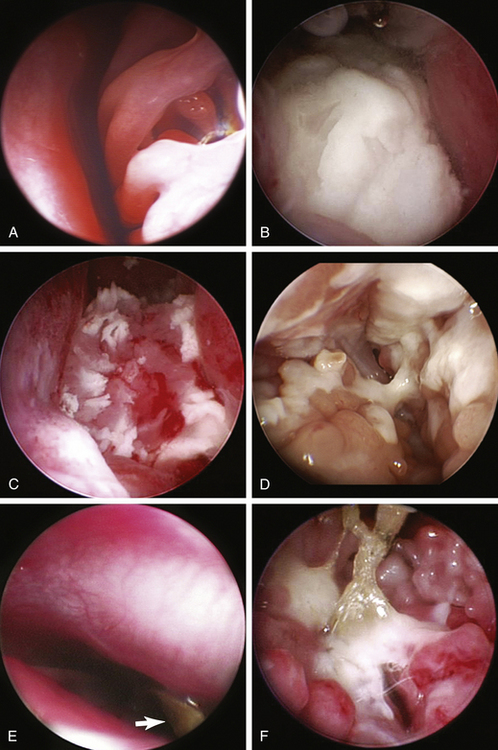
With the animal intubated and in sternal recumbency in a 10- to 20-degree head-down position, the oropharynx is packed with moistened gauze. The nasal cavities are flushed with warm sterile saline to remove any debris and excess mucus. The use of towels under the animal’s head helps prevent flooding of the table and floor. For animals more than 2 kg the 2.7-mm telescope is used; however, for smaller animals the 1.9-mm sheathed telescope is preferred. Using a sheath enables intraoperative flushing to maintain visualization; however, in small animals the naked telescope can be used with care along with intermittent syringe flushing through the nostrils. The ventral and middle nasal meatus can be exploited to examine the ventral and middle conchae. In larger animals, the endoturbinates and opening to the nasopharynx can also be seen. Care is required so that the delicate nasal turbinates that are prone to hemorrhage are not damaged (Figure 22-7, A). The telescope should be kept as medial as possible and always kept within the meatus. Even so, hemorrhage can rarely be completely avoided. Exudates, abscesses, masses, and foreign bodies can be appreciated and sampled or removed (Figure 22-7, B-F).
The recent advent of 2- and 3-mm rigid instruments also permits biopsy and débridement within the nasal or paranasal sinuses via limited surgical access.4 There are occasions when dental disease requires an extraoral approach, either alone or in conjunction with intraoral surgery, and the telescope can serve as a useful surgical aid. The extension of hypsodont roots into the nasal cavity may warrant rhinoscopy via the nostrils, as already detailed, or surgical rhinotomy. Dental abscesses affecting the maxilla may enter the paranasal sinuses, and the telescope can provide evaluation via a small (4- to 5-mm) osteotomy. Even when extensive surgical osteotomy or rhinotomy is performed, surgical access is often very limited in small herbivores, but the telescope enables detailed evaluation farther cranial and caudal to the surgical site.
Laparoscopy
For a detailed discussion of methodology the reader is referred to the dedicated laparoscopy literature as only small mammal specifics will be highlighted here.5,6,10 Laparoscopy has been shown to offer significant advantages over traditional surgical options, both in human and in veterinary medicine. In particular, laparoscopy is, with practice, faster, less traumatic, and results in less postoperative pain and a faster return to normal function. Until the advent of 2- and 3-mm human pediatric equipment, laparoscopy was limited to a single-entry system with the use of the sheathed telescope. However, multiple-entry techniques are now possible and practical for animals more than 500 to 1000 g. Indeed, laparoscopic oophorectomy is now my sterilization method of choice for female rodents and rabbits because it involves less tissue manipulation and results in less postoperative discomfort and a faster return to normal feeding and behavior.
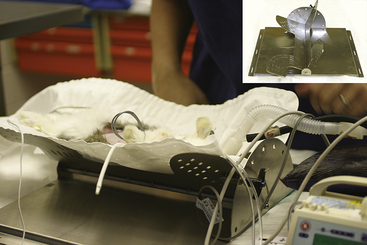
Single-entry techniques are simple and easy to perform, but the variety of instruments is limited; thus, tissue manipulation and endosurgery are rudimental. Nevertheless, visceral evaluation and biopsy are practical even in small rodents. For larger animals multiple-entry techniques using 2- and 3-mm human pediatric instruments are preferred and provide greater opportunities for endosurgery.4 There is a wider equipment selection for 3-mm instruments, and these are used with Clickline interchangeable handles connected to a radiosurgery unit for hemostasis. Access to the abdomen is achieved with the use of lightweight 3.5-mm (for instruments) and 3.9-mm (for sheathed telescope) threaded graphite cannulae. Cannula placement is determined by the organ of interest, preference of the surgeon, and anatomic nature of the animal in question (Figure 22-8). CO2 insufflation with the use of a dedicated endoflator is essential for multiple-entry techniques. Some prefer the use of a Veress needle, while others, concerned about the risk of damage to internal viscera (especially the voluminous gastrointestinal tract of small herbivores), prefer to surgically place the initial cannula or telescope. An ability to rotate the animal from dorsal to either lateral position can greatly assist with the location of ovaries, kidneys, and other dorsolateral structures. Although tilting endoscopy tables are commercially available for domesticated animals, a specifically designed and constructed small mammal tilting table is in use at the University of Georgia (Figure 22-9).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree