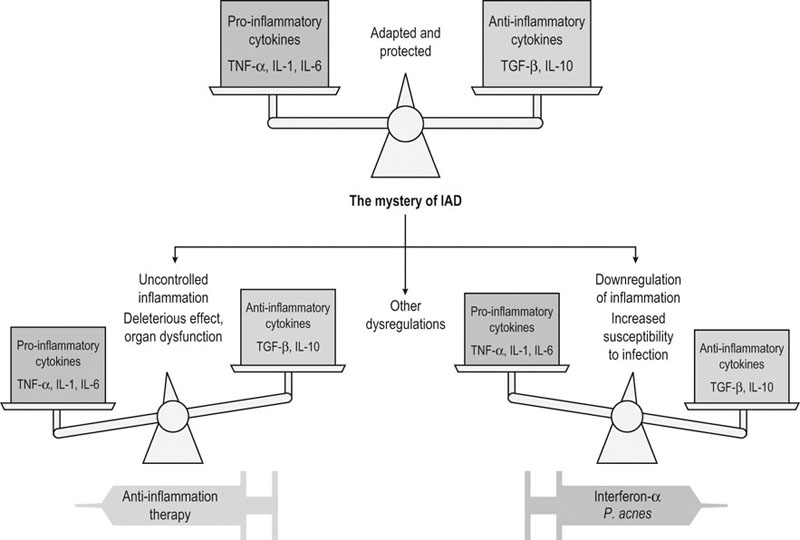
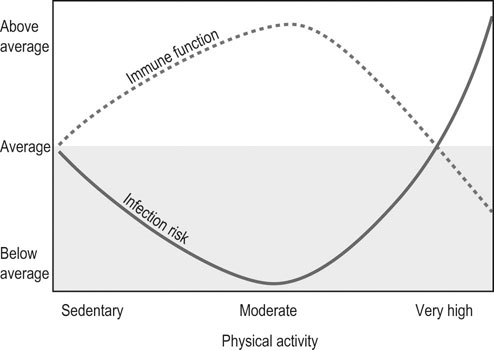
The relationship between exercise, immune function and infectious or inflammatory diseases remains poorly resolved.1–4 The difficulty of assessing this relationship is due to numerous factors, among which the complexity of the immune function, the variability of the exercise considered (which varies by equestrian discipline, intensity and duration), the population studied (sedentary, athletic, young, mature, trained or untrained), what function or what cells have been analyzed to assess the immunity, the timing of sampling used (during exercise, just after, or 1, 24 or 48 hours after); and the difficulty of dissociating the effect of the effort from other effects (psychological stresses, environment). Last but not least, it is difficult to determine the genuine clinical significance of the changes observed in some parameters of the immunity. • some cellular components: quantitative evaluation and proportion of each type of leukocyte (cells counting, identification by flow cytometry) • some functional parameters of the phagocytic cells: phagocytic capacity, oxidative burst, functional immune capacity of neutrophils by evaluating capacity of adherence, chemotaxis, ingestion and digestion of foreign substances; cytotoxicity • lymphoproliferation after non-specific (mitogens) or specific (antigens) stimulation of lymphocytes • cells response after stimulations that mimic an attack by a pathogen Recent discoveries in molecular biology have opened new prospects for studying the non-specific response of the first-line defender cells when microorganisms invade the organism. Among these findings, are pattern recognition receptors (PRR) such as Toll-like receptors (TLRs) and NOD-like receptors (NLRs) that seem to play a key role in the defense against microbes.5,6 These receptors are activated after recognizing specific pathogen-associated molecular pattern (PAMP) or damage-associated molecular pattern (DAMP). When these receptors bind and recognize their specific ligand, they induce a cascade of events leading to the activation of transcription factors, resulting in production of chemokine/cytokine, upregulation of cell adhesion molecules, phagocytic cellular infiltration, and finally clearance of the microorganisms. The study of TLRs activation by specific ligands is being investigated in equids.7–10 For example, TLR4 is located in the cellular membrane and is involved in the recognition of several bacterial signals. The TLR4 cascade is activated by the classical addition of bacterial endotoxins (lipopolysaccharide or LPS) on cultured peripheral blood mononucleated cells (PBMC) or pulmonary alveolar macrophages (PAM) and results in the production of pro-inflammatory cytokines such IL-1α, IL-6 and TNF-α. These tests, overall, evaluate the response of the phagocytic cells in front of a bacterial attack. In young racehorses in training, respiratory problems are frequently related to viral diseases (EHV4, influenza) which induces acute respiratory disease with temporary incapacity to work, and that can ‘prepare the ground’ for secondary infectious and/or inflammatory complications.11 It is therefore of paramount importance to examine the capacity of the immune system to protect against disease caused by these viruses. Polyinosinic-polycytidylic acid (poly I : C) has a structure similar to the double strand of RNA contained in some viruses and, consequently, is a tool used in research to evaluate the immune response against viral attack. It is a stimulant of the Toll-like receptor type 3 (TLR3), an intracellular receptor included in the endosome membrane and which is involved in the recognition of viral DNA/RNA. It is expressed in lymphocytes T cells, dendritic cells and phagocytes. The recognition of its ligand will mainly result in the production of INFβ. Experimental induction of the TLR3 pathway can be obtained by adding poly I : C (which has a structure similar to the viral RNA) on cultured PBMC, or PAM or bronchial epithelial cells (Fig. 45.1). Current research is aimed at studying the response of PBMC, PAM and bronchial epithelial cells to specific stimulation of TLR with synthetic ligands. The cells are put in a culture medium and stimulated by LPS (specific of TLR4), poly I : C (specific of TLR3), and other specific ligands for the different TLRs (Table 45.1). The cytokines in the supernatant are then analyzed (when ELISA homologous for the horses is available) and the white cells are recovered for extraction of RNA and transcriptomic study (when ELISA are not available). These methods have shown a probable decrease of resistance to virus 24 hours after an exercise to fatigue in Thoroughbred horses.10 Table 45.1 Toll-like receptors and some of their specific experimental ligands Epidemiological studies carried out in man have led to the following observations: • Moderate regular physical activity confers a protection against chronic inflammatory diseases (cardiac, type 2 diabetes, chronic obstructive pulmonary disease) and reinforces the resistance of the subjects to the infectious viral and bacterial diseases. This is especially true in the elderly.3,4 • Intense physical activity, and particularly a competition, induces an increased (+150%) susceptibility to upper airway respiratory diseases, just after the exercise. Moreover, intense exercise seems to reactivate latent infections.3,12,13 (see Fig. 45.2). • The susceptibility to infections of the upper airway is greater in ‘overtrained’ subjects.12 • There is a much higher prevalence of asthma and bronchitis in human athletes, especially in marathon runners, than in the sedentary population. Air quality and characteristics (chemical, biological and physical) as well as high level of ventilation are thought to be favoring factors.4 In horses, epidemiological studies have also shown an increased susceptibility to infectious disease in young racehorses, despite appropriate vaccination.11 Another characteristics of racehorses regularly trained and involved in competition is the fact that they frequently suffer from chronic inflammation of the airway, a syndrome commonly known as inflammatory airway disease (IAD).14 The primum novens and the mechanisms of occurrence of IAD remain poorly defined to date. Most probably, the problem is related to an inappropriate response of the innate immune system. Dysregulation of immunity in IAD could be due either to an uncontrolled chronic inflammation or, on the contrary, a subdued inflammatory response, favoring the occurrence of secondary microbial and fungal infections. The fact that some scientists propose to treat IAD by anti-inflammatory drug administration while others recommend to control it by giving stimulants of immunity gives an idea of the extent of the lack of knowledge on this syndrome (Fig. 45.3).
Exercise and immunity in horses
Introduction
Assessment of immunity
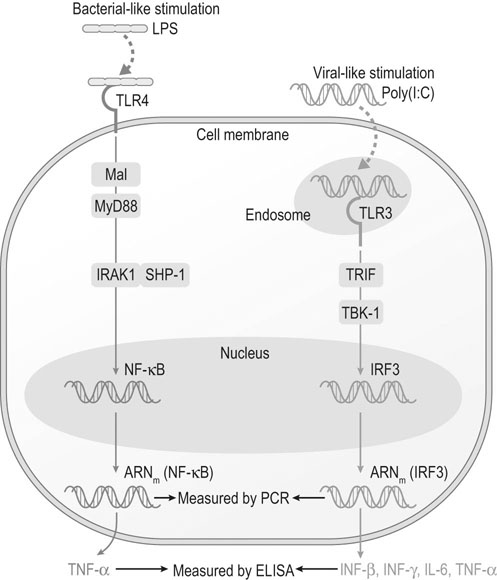
TLR
LIGANDS
TLR-1&2 (heterodimer)
Peptidoglycan, lipoproteins, zymosan
TLR-1&6 (heterodimer)
Peptidoglycan, lipoproteins, zymosan
TLR-3
dsRNA
TLR-4 (dimer)
LPS (Gram-), lipoteichoic acid (Gram+)
TLR-5
Flagellin
TLR7
ssRNA
TLR-8
G-rich oligonucleotide
TLR-9
Unmethylated CPG DNA
Epidemiologic data
Exercise and immunity in horses