CHAPTER 23 Examination of the Urinary Sediment
SPECIMEN COLLECTION
Urine must be properly collected to ensure that urinalysis results are reliable. It can be collected in four ways (Table 23-1): catching a sample during urination, expressing the bladder, catheterization, and cystocentesis.1 Cystocentesis and catheterization are the preferred methods because both provide optimal samples for all aspects of urinalysis by avoiding contamination from the lower genital tract and external genitalia. Although it may be easier to express the bladder or catch a sample during urination, urine collected in these ways may be limited for analysis (particularly for a bacterial culture). Urinalysis is usually performed on preprandial, morning samples, because they tend to be the most concentrated, thus increasing the chance of finding abnormalities.
Table 23-1 Methods of Urine Collection with Advantages and Disadvantages of Each
| Method | Advantages | Disadvantages |
|---|---|---|
| Voided | Noninvasive, does not require special expertise, can be collected by owner | Contaminated by urethra and prepuce, therefore can have increased RBC and/or WBC due to urethral or prepucial inflammation and is not recommended for culture when cystitis is suspected |
| Expressing the bladder | Noninvasive, requires only minimal expertise, can be performed at time of examination | Contaminated by urethra and prepuce, therefore can have increased RBC and/or WBC due to urethral or prepucial inflammation and is not recommended for culture when cystitis is suspected |
| Catheterization | Semi-invasive, requires less expertise than cystocentesis, can be performed at time of examination | Some contamination, but less than voided or expressed urine, can contribute to development of cystitis after catheterization |
| Cystocentesis | Best for urine culture, less likely to cause cystitis than catheterization, can be performed at time of examination | Invasive but seldom causes cystitis, requires greater expertise than other methods of urine collection |
Courtesy R.D. Tyler and R.L. Cowell.
MICROSCOPIC EXAMINATION OF URINE SEDIMENT
In order to semiquantitatively test the formed elements in urine, a standard volume, usually 5 ml, is centrifuged to obtain the sediment on every sample. Then 5 ml of a well-mixed sample is placed in a graduated, conical centrifuge tube and centrifuged for 3 to 5 minutes at approximately 100 G (about 1000 to 2000 rpm depending upon the radius of the centrifuge). The procedure should be standardized for a particular centrifuge to yield uniform results. Some centrifuges such as the Clay-Adams Triac (Becton Dickinson, Parsippany, NJ) are precalibrated to provide the proper force over sufficient time to completely sediment the formed elements in the urine. After centrifugation, the volume of sediment is recorded. The supernatant is gently poured out, leaving about 0.3 ml of urine adhering to the sides of the tube. This urine is allowed to run down the inside of the centrifuge tube, and the sediment is resuspended by gently flicking the bottom of the centrifuge tube.2
URINARY SEDIMENT
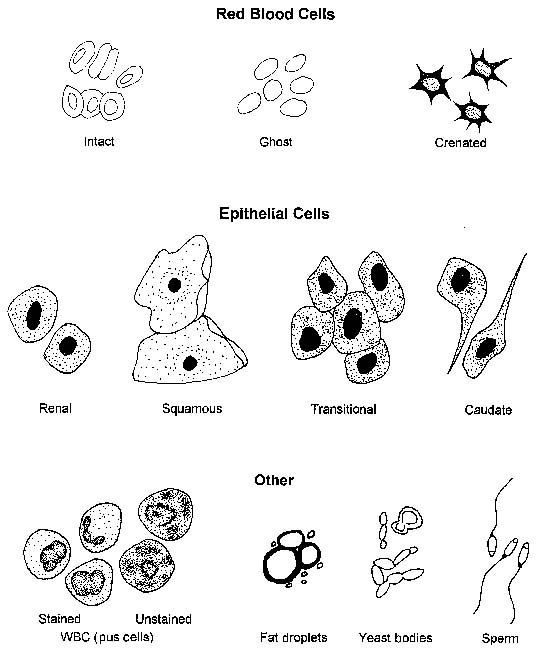
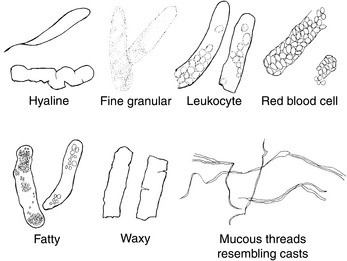
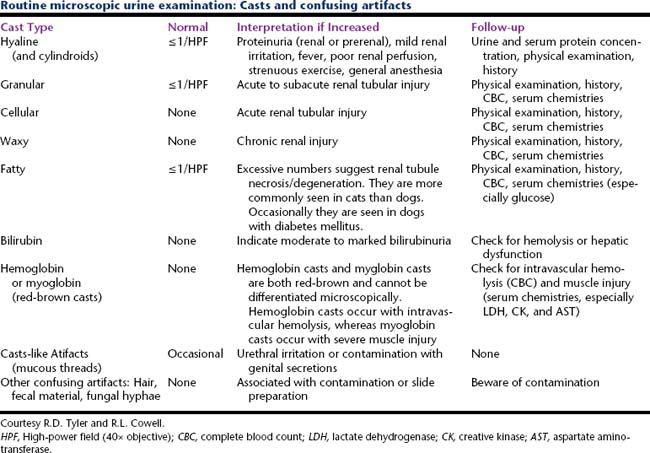
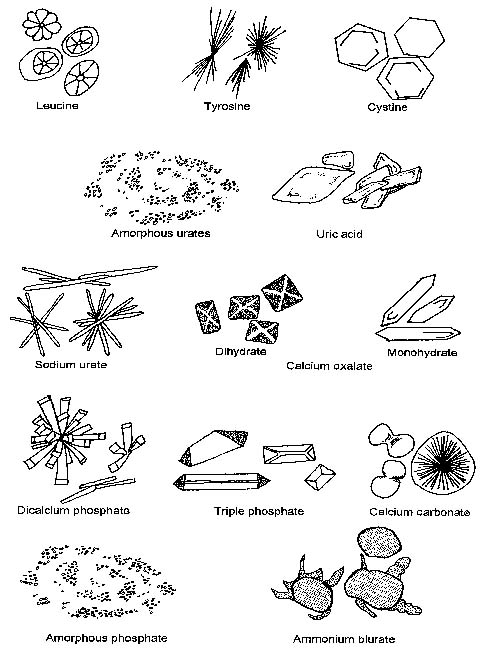
Cells (Figure 23-1, Table 23-2), microorganisms (Table 23-2), casts (Figure 23-2, Table 23-3), crystals (Figure 23-3, Table 23-4), fat, and contaminating substances may be found in urinary sediment.
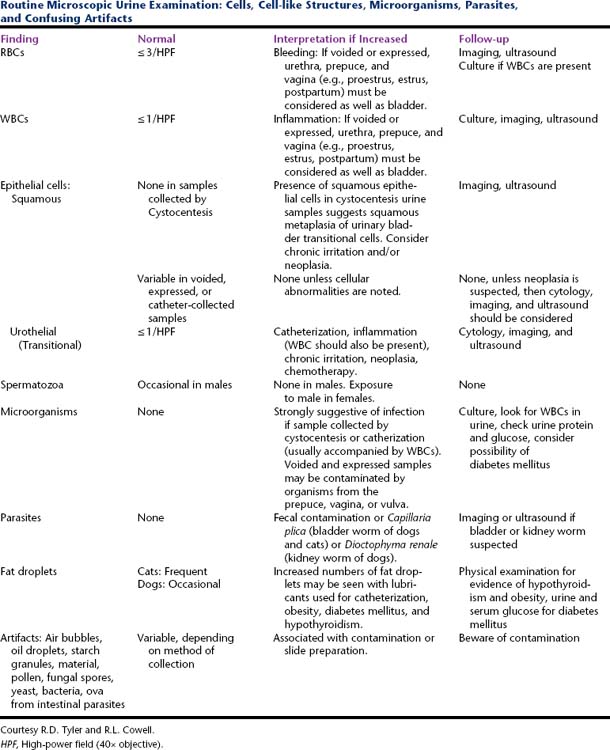
Table 23-2 Routine Microscopic Urine Examination: Cells, Cell-like Structures, Microorganisms, Parasites, and Confusing Artifacts
Red Blood Cells
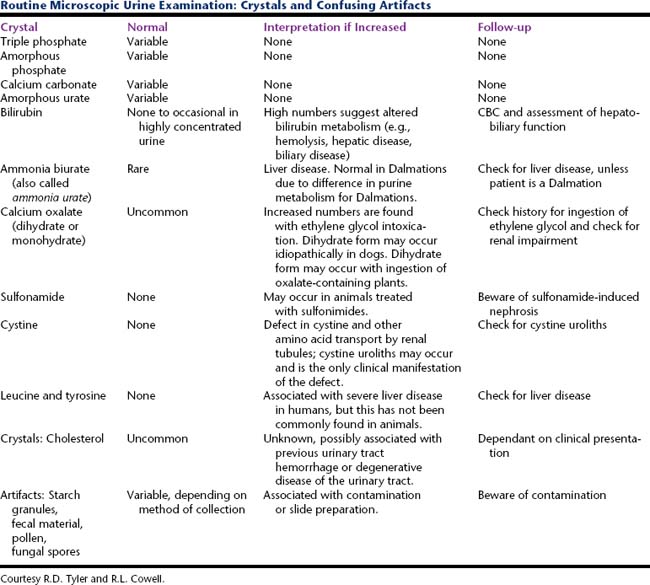
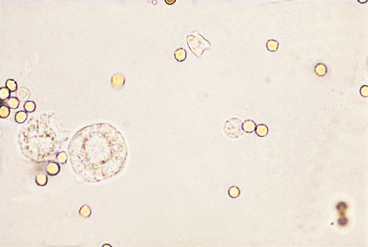
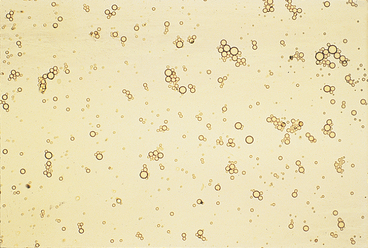
Urine sediment normally contains fewer than 2-3 RBCs/HPF. RBCs are small cells that may have several different appearances, depending on the urine concentration (e.g., specific gravity) and the length of time between collection and examination (Figures 23-4 to 23-9). In fresh samples that have intermediate specific gravities, RBCs usually have smooth edges and are yellow to orange. They can be colorless if their hemoglobin diffused while standing. In concentrated urine, RBCs shrink and crenate (see Figures 23-4 and 23-9). Crenated RBCs have ruffled edges and are slightly darker. Extremely crenated RBCs may even appear granular because of membrane irregularities. In dilute or alkaline urine, RBCs swell and may lyse. Swollen RBCs have smooth edges and are pale yellow or orange. Lysed RBCs may be colorless rings (shadow or ghost cells) that vary in size, but, especially when due to marked alkalinity, they usually dissolve and cannot be identified by microscopic examination.
RBCs must be differentiated from WBCs in urine sediments. This is easy on stained sediment smears because RBCs are anucleate, but differences in size and internal structure must be used to differentiate between them on unstained smears. RBCs that maintain their biconcave shape can be easily recognized by their dark central area (see Figure 23-5), but those that shrink or swell in response to urine osmolality may not demonstrate the typical biconcave shape. These RBCs are differentiated from WBCs based on size (approximately half the diameter of a WBC; see Figure 23-6) and lack of internal structure. Although RBCs have no nuclei, they should not be confused with fat globules or yeast. RBCs do not vary much in size, are yellow to orange, and tend to settle onto a slide. Fat globules (Figure 23-10) vary markedly in size and are light green and usually found just under the coverslip. (This last feature causes them to be in a different plane of view from other urine sediment elements.) Yeast organisms are rarely found in fresh urine from dogs and cats, but are typically seen in aged samples with overgrowths of contaminants. When present, yeast organisms are more variable in size than RBCs, and budding can usually be found on some of the organisms.
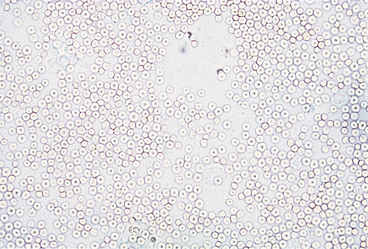
White Blood Cells
Very few WBCs are present in urine from animals without urinary or genital tract disease. WBCs are about twice the size of RBCs and smaller than epithelial cells (see Figures 23-6 to 23-8). They are round and granular and sometimes brownian movement of the cytoplasmic granules in the neutrophils is seen. The polymorphic neutrophil nuclei can usually be seen in stained sediments and with phase-contrast microscopy and may occasionally be seen with reduced illumination bright field microscopy. WBCs are usually in very low numbers in urine (i.e., 0 to 1/HPF); more than 2 to 3 WBCs/HPF indicates inflammation somewhere in the urinary or genital tract. When increased numbers of neutrophils are found in urine sediment, even if bacteria are not found by microscopic examination, the urine sample should be cultured because microscopic examination is much less sensitive than culture to detecting bacteria in urine.
Epithelial Cells
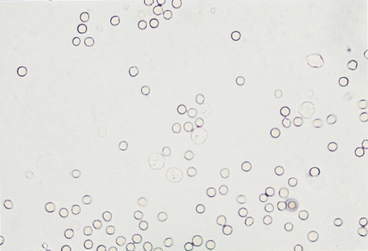
Squamous Epithelial Cells
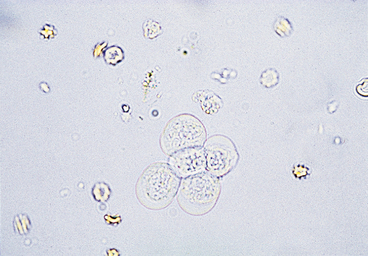
Squamous epithelial cells, which are derived from the distal urethra, vagina, vulva, or prepuce, are occasionally found in voided samples (Figure 23-11) and are usually not considered significant. They are the largest of the epithelial cells and the largest cells found in urine sediment; they appear flat and often have straight edges and obtuse, angular corners. They usually have a small, round nucleus, although occasionally a nucleus cannot be seen. Squamous epithelial cells are usually not found in samples obtained by cystocentesis. Cells with squamous features can be found in the urine sediment of male dogs with conditions that cause squamous metaplasia of the prostate and occasionally in the sediment of those with urothelial carcinomas (i.e., transitional cell carcinomas) in which there is also squamous metaplasia. In the latter condition, many other features suggestive of epithelial neoplasia, including extreme numbers of epithelial cells with marked pleomorphism, are found.
Urothelial (Transitional) Epithelial Cells
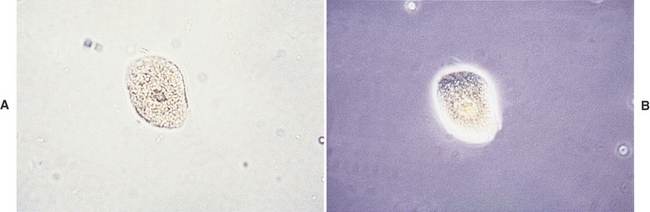
Urothelial, or transitional, epithelial cells come from the proximal urethra, bladder, ureters, renal pelvis, and renal tubules (see Figures 23-8 and 23-9). They vary in size depending upon their origin. Those originating in the proximal urethra and bladder are the largest, are round to elliptical, and have granular cytoplasm and variably sized nuclei. Epithelial cells of the ureters and renal pelvis are smaller, are round to caudate, and have granular cytoplasm. Renal tubular epithelial cells are small, round cells with a distinct, round nucleus. They have granular cytoplasm that may contain a few small to large fat vacuoles. Fatty renal tubular cells are especially common in cat urine. Renal tubular epithelial cells are only slightly larger than WBCs and are differentiated from WBCs by their round nuclei. A few epithelial cells, especially urothelial cells (i.e., 0 to 1/HPF), are found in sediment from normal animals due to the sloughing of old cells. An increase in epithelial cell numbers may be found in samples obtained by catheterization. Modest to marked increases in epithelial cells are found in inflammatory diseases of the urinary tract because inflammation often causes urothelial hyperplasia. Urine samples from animals with inflammation-induced urothelial hyperplasia also contain increased WBC numbers. Some chemotherapeutic agents (e.g., cyclophosphamide) may induce urothelial hyperplasia with modest pleomorphism. Many epithelial cells are found in sediment from animals with transitional cell carcinomas. Neoplastic urothelial cells usually vary markedly in size. If large numbers of epithelial cells (especially if they vary markedly in size) are found, sediment smears should be made, air-dried, stained with a hematologic stain, and evaluated cytologically.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree