Chapter 78 Ethylene Glycol
METABOLISM
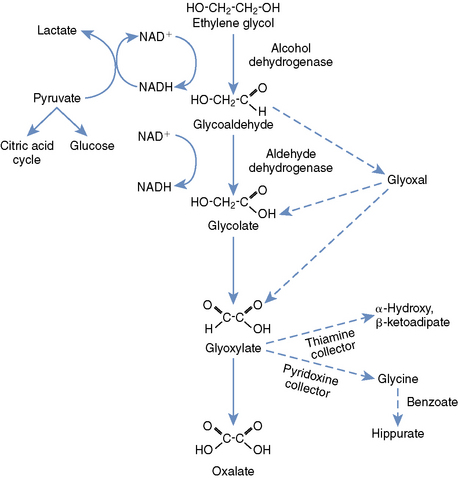
Knowledge of the metabolism of EG is paramount to understanding its toxicity and treatment (Figure 78-1). Metabolism is accomplished primarily in the liver, with a small contribution from the kidneys and stomach. Alcohol dehydrogenase (ADH), an important therapeutic target, converts EG to glycoaldehyde and organic acids. The glycoaldehyde is then converted to glycolate (glycolic acid [GA]), then glyoxylate (also known as glyoxylic acid). Because the metabolism of GA is rate limiting, this metabolite achieves much higher concentrations in the blood than any of the others. Glyoxylate is converted primarily to oxalate, but additional end products include glycine, formic acid, hippuric acid, oxalomalic acid, and benzoic acid. The oxalate formed from glyoxylate combines with calcium to form the characteristic calcium oxalate crystals that are deposited throughout the body, but primarily in the kidneys.1,3
DIAGNOSIS
The first clinicopathologic finding seen in patients with EG ingestion is a rise in the osmolal gap (OG) (Box 78-1). This can occur as early as 1 hour after ingestion in cats and dogs and typically peaks by 6 hours in dogs. The OG remains elevated for approximately 18 hours after ingestion of EG. The OG correlates well with EG concentration in both humans and dogs, and in human intoxications the deviation from a particular laboratory’s “normal” OG (typically around 10 mOsm/kg H2O; up to 150 mOsm/kg with EG toxicity) can be multiplied by 6.2 to estimate EG concentration in mg/dl. Data from dogs again show a high correlation between OG and EG concentrations, but this formula appears to be less reliable in this species. Serum osmolality should be measured by freezing point depression to accurately calculate the OG.1,3-6
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree