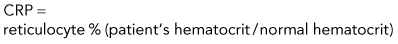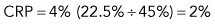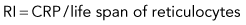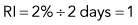3 Erythrocyte Disorders
Anemia Diagnosis
Anemia is the most common erythrocyte (red blood cell [RBC]) disorder. Anemia can cause various clinical signs (e.g., weakness, lethargy, exercise intolerance, heart murmur, hemoglobinuria, pica, shock, death) or may be subclinical and detected only during a general diagnostic workup. Causes of anemia are listed in Box 3-1. An approach to anemia diagnosis is outlined in Box 3-2. A detailed description follows in specific sections.
Box 3-1 Common Types of Anemia
Nonregenerative Anemia
Anemia of inflammatory diseases
Anemia of chronic renal disease
Anemia of chronic hepatic disease
Hypothyroidism and hypoadrenocorticism
Nonregenerative immune-mediated hemolytic anemia
Bone marrow necrosis/inflammation
Macrophage proliferative disorders
Drug-Induced Hematologic Dyscrasia
Feline immunodeficiency virus (FIV)
Box 3-2 Approach to Anemia Diagnosis
I Determine Severity of the Anemia (see text)
II Determine Bone Marrow Responsiveness
III Regenerative Anemia Diagnosis
IV Nonregenerative Anemia Diagnosis
The presence and severity of anemia is usually documented by determining a packed cell volume (PCV; hematocrit) (see Chapter 2). Hemoglobin concentration (Hgb) and RBC count are equivalent indicators of the presence and severity of anemia, but for simplicity, only the PCV is used in the following discussions. Exceptions to the rule that PCV, Hgb, and RBC count decrease in parallel in anemia occur if the blood has many, very small (microcytic hypochromic) RBCs or many, very large (macrocytic hypochromic) RBCs.
Hydration status and blood volume affect assessment of the severity of anemia. Clinical dehydration (i.e., hemoconcentration) reduces plasma volume, which causes a relative increase in PCV (relative polycythemia). Dehydration may mask detection of a mild to moderate anemia, most often early in diagnosis. An animal’s hydration status must be normal before the PCV properly reflects the degree of anemia. After hemorrhage, for example, it may take 1 to 2 days to refill the blood volume, based on intake of water and other fluids, and then the PCV will reflect the severity of the anemia. Hydration status is usually assessed by considering plasma protein (PP) concentration and PCV in combination. Disease-induced changes in PP or PCV (e.g., anemia or hypoproteinemia) interfere with the use of these values in evaluation of hydration status. Severe hypoproteinemia may be due to protein loss via hemorrhage, intestinal disease, glomerular disease, or the like (see Chapter 12). PP determination is not a sensitive test of dehydration but is easily performed and commonly available. Additionally, the spleen can affect PCV (and Hgb and RBC count) because it contains 20% to 30% of the RBC mass, and splenic contraction causes rapid changes in distribution by releasing a concentrated bolus of stored RBCs, more so in the dog than cat. The splenic effect is immediate and seen mainly in normal animals. To a lesser extent, the spleen can relax to store RBCs, removing them from circulation (e.g., under anesthesia).
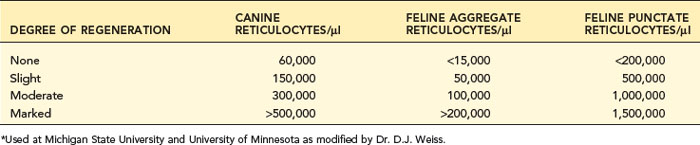
After documenting the presence of anemia and grading its severity, one should evaluate the bone marrow’s erythroid production to determine if the bone marrow appears to be contributing to the anemia or responding appropriately to decreased RBC mass. A key question in anemia is, “Is the bone marrow responding as expected to the anemia, or is it the cause of the anemia?” Bone marrow effective erythropoiesis is primarily judged by its release of reticulocytes (Tables 3-1 and 3-2). Bone marrow function should respond normally in blood loss or hemolytic anemia, and reticulocyte production should be increased in proportion to the severity of the anemia. Anemia with appropriately increased erythropoiesis is classified as regenerative anemia (see Box 3-2). A nonregenerative anemia is indicated by finding clearly less than an expected reticulocyte response for the time after onset of an anemia and the severity of the anemia. A moderate to severe anemia should have a maximal response 3 to 7 days after onset of the anemia.
TABLE 3-1 DEGREE OF ERYTHROID REGENERATION IN ANEMIA
| DEGREE OF STIMULATION | Percentage of Reticulocytes | |
|---|---|---|
| DOGS | CATS* | |
| Normal | 1 | 0-0.4 |
| Slight | 1-4 | 0.5-2 |
| Moderate | 5-20 | 3-4 |
| Marked | 21-50 | 5+ |
* Indicates the percentage of aggregate reticulocytes in cats.
From Perman V, Schall WB: Diseases of the red blood cells. In Ettinger SJ, editor: Textbook of veterinary internal medicine: diseases of the dog and cat, ed 2, vol 2, Philadelphia, 1983, WB Saunders.
Determining Erythroid Regeneration
Reticulocyte Evaluation
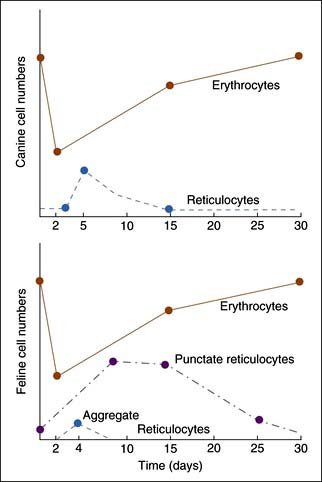
Reticulocyte enumeration in blood is the most consistent way to evaluate the strength of erythropoiesis, but one must consider time after onset of the anemia (Figure 3-1) and magnitude of the reticulocytosis (see Table 3-2) during interpretation. A reticulocyte count should be performed when the PCV is less than 30% in dogs and less than 20% in cats.
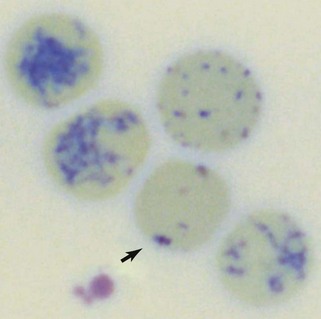
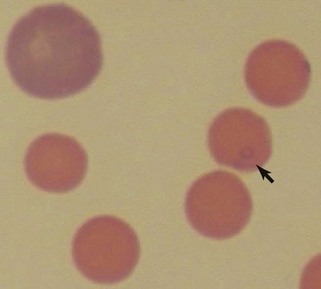
Reticulocytes are immature RBCs released in increased numbers from normal bone marrow in response to anemia. Reticulocytes have ribosomes (ribonucleic acid [RNA]) for continued hemoglobin synthesis. Ribosomal material appears as dark-blue granules when stained with new methylene blue (NMB) (Figure 3-2). Feline reticulocytes should be subdivided into aggregate and punctate forms, because the cat is unique in having large numbers of punctate reticulocytes. Reticulocytes are reported as absolute reticulocyte numbers per microliter of blood, reticulocyte index (RI), reticulocyte percentage, and corrected reticulocyte percentage, or they can be estimated by inspection of the number of polychromatophilic RBCs on blood smears (see later). Reticulocytes are larger (i.e., macrocytes) and have a lower hemoglobin concentration than mature RBCs. Therefore documenting increased numbers of macrocytic hypochromic erythrocytes usually reflects reticulocytosis and bone marrow responsiveness.
Automated Reticulocyte Counting
Various veterinary hematology instruments use different reticulocyte stains for an automated reticulocyte count. Sensitivity of reticulocyte detection varies among instruments and methods.17 The Advia 2120 and Sysmex XT-2000iV reticulocyte counts are approximately correct for canine reticulocytes and correlate best with feline aggregate reticulocytes (see Figure 2-5). Sysmex’s reticulocyte staining is slightly more sensitive and detect about 6% punctate reticulocytes in its automated reticulocyte count in addition to aggregate reticulocytes. The Sysmex automated reticulocyte count still should be considered mainly an aggregate reticulocyte count. Feline punctate reticulocytes must be determined by manual evaluation of reticulocyte stained smears to identify prominent changes in punctate reticulocyte numbers. The LaserCyte (IDEXX Laboratories) uses NMB to stain reticulocytes and is less sensitive than the Advia or Sysmex instruments. The LaserCyte has a negative bias and proportional error compared to manual or automated (Advia) methods. Instrument-specific values for considering LaserCyte reticulocyte results nonregenerative (<35,000/µl) or regenerative (>75,000/µl) were suggested. A cutoff for canine regenerative anemia is often stated to be greater than 60,000/µl, but 60,000/µl should instead be considered a mean reference value and not maximum value. Reference values for Advia automated canine reticulocyte counts are 11,000 to 111,000/µl and 0.1% to 1.5%.29 Reference values for Sysmex XT automated canine reticulocyte counts are 19,400 to 150,100/µl and 0.3% to 2.4%.30 With availability of routine automated reticulocyte counts, many nonanemic dogs are noted to have many more than 60,000 reticulocytes/µl. Excitement and splenic contraction may cause reticulocytosis. The most common error in interpretation of reticulocytes is to conclude an anemia is regenerative based on slight increases in one of the ways of reporting reticulocytes.
Reticulocyte volume can be measured and used in cases of iron deficiency anemia to document response to treatment. Reticulocytes are smaller in iron deficiency but increase in volume with iron therapy. For example, after experimental iron replacement in iron-deficient dogs, the mean cell volume of reticulocytes (MCVr; Advia 2120) increased from 65 fl before iron treatment to 82 fl after 11 to 12 days of iron supplementation, and hemoglobin per reticulocyte (cell hemoglobin reticulocyte, CHr) increased from about 16 up to 22 pg.9
Absolute Reticulocyte Count
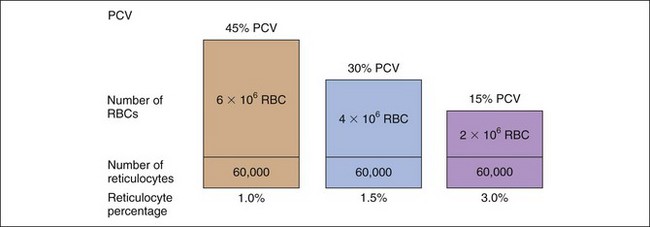
The percentage of reticulocytes may be reported alone (see Table 3-1), but this can be misleading because percentage is a ratio of reticulocytes to mature RBCs. In anemia, the mature RBCs are variably reduced, thus the reticulocyte percentage overestimates the hematopoietic response (Figure 3-3). The absolute reticulocyte count is calculated by multiplying the reticulocyte percentage by the RBC count, so it adjusts the reticulocyte percentage for the severity of the anemia. Absolute reticulocyte count is the more consistent indicator of bone marrow production and is recommended as the best single indicator of regeneration in the dog and cat (see Table 3-2). If an RBC count is not available, a corrected reticulocyte percentage (CRP) and RI (also termed reticulocyte production index) can be determined.
Reticulocyte Index and Corrected Reticulocyte Percentage
Discussion of CRP and RI is included here, but only to illustrate some principles. RI and CRP are not recommended for clinical diagnosis interpretation. The CRP is adjusted for the degree of anemia (Box 3-3). The RI is further adjusted for the life span of the canine reticulocyte in peripheral blood. With increasingly severe anemia, reticulocytes are released earlier from the bone marrow and live longer in blood before maturing into RBCs. The longer life span increases the reticulocyte percentage, but that portion is not the result of release of increased numbers of reticulocytes from bone marrow. Feline reticulocyte life spans in blood with increasingly severe anemia are not well determined, so the RI is not recommended for cats.
Box 3-3 Steps in Calculation of the Reticulocyte Index
Step 1: Corrected reticulocyte percentage (CRP)
Example: Dog with packed cell volume (PCV) of 22.5% and 4% reticulocytes
| HEMATOCRIT | EXPECTED RETICULOCYTE LIFE SPAN (DAYS) |
|---|---|
| 45 | 1.0 |
| 35 | 1.5 |
| 25 | 2.0 |
| 15 | 2.5 |
An example is as follows (see Box 3-3): A dog with a PCV of 22.5% and 4% reticulocytes had an RI of 1 that denoted an inadequate regenerative response. The reticulocyte percentage of 4% did not indicate a fourfold increase in reticulocyte production.
Canine Reticulocyte Response
Canine reticulocytes normally mature to RBCs in about 1 day after release into the blood. As a result of this rapid maturation, reticulocytes are primarily aggregate reticulocytes (i.e., they contain a large mass of precipitated RNA with few punctate forms). Canine reticulocytes are not subdivided because the small number of punctate reticulocytes in the dog is clinically insignificant given the great variability and error potential in microscopic counts. Most laboratories do not count RBCs with only one or two individualized granules as reticulocytes, to be certain not to include RBCs with granular artifact or precipitate. Canine reticulocytes approximately equal polychromatophils seen on Wright-stained blood smears, though different stains vary in how well they display polychromatophils. Some guidelines assess the degree of activity of bone marrow according to the percentage of reticulocytes (see Table 3-1). This has been modified to provide absolute reticulocyte numbers to judge the magnitude of bone marrow regeneration (see Table 3-2).
Feline Reticulocyte Response
Cats vary from dogs in having large numbers of punctate reticulocytes both normally and in regenerative anemias. A total feline reticulocyte count does not provide the same interpretation as it does in dogs. A feline reticulocyte count should be subdivided into punctate and aggregate reticulocytes to reflect significant differences in stages of maturation (see Figure 3-2). Aggregate reticulocytes mature rapidly, in about half a day, into punctate reticulocytes. Punctate reticulocytes mature slowly over 10 to 12 days and thus accumulate in blood in much greater numbers than do aggregate reticulocytes. Reports that do not identify the type of reticulocyte counted in a cat are worthless! The duration of a regenerative anemia may be suggested by the pattern of reticulocyte response. For example, at about 4 days into a regenerative response, the aggregate reticulocyte response peaks and punctate reticulocytes are still relatively low (see Figure 3-1). Thereafter, aggregate reticulocytes decrease and punctate reticulocytes continue to increase. Polychromatophils on feline Wright-stained blood smears reflect the number of aggregate reticulocytes (Figure 3-4).
In persistent hemorrhage or a hemolytic process, the combined punctate and aggregate reticulocyte count (mainly punctate) may approach 100%. This makes the performance of reticulocyte counts tedious. Even with more normal numbers of reticulocytes, no Heinz bodies, and little or no stain precipitate, microscopic reticulocyte determinations are imprecise. Flow cytometric analysis of feline blood in which the RNA of reticulocytes was stained with thiazole orange was a more sensitive and reliable assay of feline reticulocytes than manual microscopic evaluation.18 Reference intervals for 38 clinically normal cats by this thiazole orange method were 0.1% to 0.5% (8500 to 42,000/µl) for aggregate reticulocytes and 2% to 17% (22,500 to 1,270,000/µl) for punctate reticulocytes.16 Manual reference values should be similar.
Polychromasia
Polychromasia denotes an increased number of polychromatophils observed on Wright-stained blood smears. Canine polychromatophils are equivalent to reticulocytes, and polychromasia indicates reticulocytosis. These are larger than mature RBCs and have slightly bluer staining because of ribosomes in the cytoplasm (see Figure 3-4). Polychromatophilic refers to multiple (“poly”) colors with the orange staining of hemoglobin plus the blue staining of RNA. Feline aggregate reticulocytes appear as polychromatophils, but punctate reticulocytes do not. Therefore polychromasia on feline CBC reports reflects only aggregate reticulocyte numbers, whereas polychromasia on canine blood smears reflects total reticulocyte numbers. Increased polychromasia in both species denotes active bone marrow erythropoiesis 3 to 7 days earlier. The magnitude of the polychromasia reflects the strength of the erythropoiesis.
Macrocytosis
Larger than normal RBCs (i.e., macrocytes) are documented by the mean corpuscular volume (MCV) or by RBC cytograms and RBC volume histograms (see Figures 2-3 to 2-5). Reticulocytosis is the most frequent cause of macrocytosis, especially at 4 to 5 days after the onset of anemia, but mature macrocytes can also be released during accelerated erythropoiesis. Tvedten believes macrocytosis—and especially the percentage of macrocytic hypochromic RBCs—is a more sensitive indicator of increased erythropoiesis late (e.g., 7 to 14 days) in the regenerative response, when polychromasia and reticulocytosis are declining.22
Nucleated Red Blood Cells, Basophilic Stippling, Howell-Jolly Bodies
Circulating NRBCs are reported as the number of NRBCs per 100 WBCs or absolute number of NRBCs per volume of blood (see Chapter 2). The absolute number is more consistent for interpretation because severe neutropenia, such as in sepsis and heat stroke, can exaggerate the number of NRBCs/100 WBCs. NRBCs may be released in regenerative anemia, but NRBC numbers inconsistently reflect bone marrow erythropoiesis in dogs and cats. NRBC release into blood may occur independently of increased erythropoiesis in situations such as splenic disease, extramedullary hematopoiesis, heat stroke, sepsis, lead poisoning, hyperadrenocorticism, leukemia, and various bone marrow diseases.
Basophilic stippling is most often associated with regenerative anemias and is no cause for alarm. Basophilic stippling may be caused by lead poisoning but is neither a specific nor a sensitive method for diagnosing lead poisoning in dogs in our experience. Toxicologic testing should be used to evaluate suspected lead poisoning (see Chapter 17 and Appendix I).
Siderocytes, Sideroblasts
Siderocytes are abnormal RBCs with basophilic granules (i.e., Pappenheimer bodies) resembling basophilic stippling in Wright-stained blood smears. Prussian blue stains the iron within Pappenheimer bodies but not granules associated with basophilic stippling. Sideroblasts are nucleated erythroid cells in blood or bone marrow with iron-positive granules. Although some sideroblasts are considered normal, increased numbers and abnormal sideroblasts are considered abnormal. Abnormal sideroblasts have more numerous and larger granules that may form a ring around the nucleus (i.e., ringed sideroblasts). Siderocytes and abnormal sideroblasts indicate abnormal erythropoiesis (i.e., dyserythropoiesis). Chloramphenicol therapy, sideroblastic anemia, and some types of myelodysplastic syndromes are causes of increased siderocytes in blood and abnormal sideroblasts in blood or bone marrow.24
Morphologic Classification of Anemia
Classification of Anemia by Use of Erythrocyte Volume and Hemoglobin Concentration
Morphologic classification of anemia traditionally uses RBC indices (i.e., MVC, MCHC). MCV and MCHC are mean values of all RBCs. Mean values are not a sensitive indicator of small to medium increases of macrocytic hypochromic or microcytic hypochromic RBCs. DiNicola et al. reported that only 8% of blood samples of 6752 dogs with regenerative anemia had both increased MCV and decreased MCHC despite the principle that regenerative anemia has increased numbers of macrocytic and hypochromic RBCs, which is true and important in diagnosis.6
The identification of increased numbers of macrocytic and hypochromic RBCs is best performed by the Advia automated hematology system. The Advia instrument measures the volume and hemoglobin concentration of each individual RBC in its sample and displays each erythrocyte by size and hemoglobin concentration (see Figure 2-4). In this way, even a few abnormal RBCs can be identified on the RBC cytogram. A better estimate of the number (%) of abnormal RBCs may be made from the RBC volume and Hgb concentration histograms or from precise numbers of cells in the nine boxes of the RBC cytogram that are available from a research screen. The morphologic classification of anemia works best with the Advia instrument and unfortunately poorly with only the MCV and MCHC results that are provided by other instruments. Use of MCV and MCHC alone in diagnosis often gives incorrect classifications of the true changes in the anemia, and this can cause incorrect diagnosis.