Chapter 24 Laparoscopic and thoracoscopic techniques provide minimally invasive access to the abdominal and thoracic cavities, respectively. They allow diagnostic evaluation of various organs and tissues and provide an ever growing armamentarium of therapeutic interventions to be carried out through small port incisions. The need for “open” surgical access to body cavities is thus obviated, resulting in less pain14,47 and a more rapid return to normal activity,12,29 as well as a lower rate of postoperative wound infection in clean and clean-contaminated wounds.30 Other advantages have been demonstrated in studies of these techniques when used on human beings, although many of these techniques remain to be studied in veterinary patients. Diagnostic angiography has played an important role in human radiology since percutaneous arterial access was described by Seldinger in the 1950s.41 Since that time, advances in imaging technology and medical devices have transformed a once purely diagnostic modality into a widespread and evolving therapeutic subspecialty of human medicine with constantly expanding potential. Interventional radiology is defined as the use of these contemporary imaging modalities to gain access to different structures throughout the body to deliver therapeutic materials for a variety of conditions. Interventional endoscopy uses endoscopes instead of, or in addition to, fluoroscopy to facilitate access into different structures, most commonly through natural orifices. Interventional radiology and interventional endoscopy techniques have provided therapeutic options for diseases once deemed untreatable and have even become considered the standard of care for a variety of human conditions. Angulation of the tip of the telescope dictates the direction of the field of view. The 0 degree telescope will provide an image of what lies directly in front of the tip of the scope, which is adequate for most abdominal procedures. The 30 degree telescope provides a view that is offset by 30 degrees. Although these telescopes are somewhat more difficult to manipulate initially, they are helpful in that rotation of the light post (and therefore the telescope) allows a greater field of view (Figure 24-1). In addition, angled telescopes allow the operator to view spaces that might be difficult to maneuver the scope into otherwise, such as around the lung, heart, or liver lobes. This feature is especially helpful when procedures are performed in the thoracic cavity, making a 30 degree telescope an ideal choice for thoracoscopy. Telescopes with more acute angulations are available but are used less commonly. In the past, many veterinarians viewed endoscopic images through the eyepieces of flexible and rigid endoscopes. Although this approach may be adequate for some indications such as otoscopy, it is not practical for performance of minimally invasive surgical procedures. Video endoscopy therefore is mandatory when laparoscopy and thoracoscopy are performed, to allow the surgeon unimpeded viewing and the freedom to move around the patient during the procedure. The basic equipment needed for laparoscopy and thoracoscopy is usually housed on a multitiered cart that is often referred to as the “tower” (Figure 24-2). Basic components mounted on the tower include the monitor, camera control box, light source, and insufflators, along with data recording devices. The image that is transmitted through the lenses of the telescope is captured by the camera and turned into a video image. The camera attaches to the head of the telescope and has a cord that feeds into the camera control box housed on the tower. The quality of the endoscopic video camera depends on the camera control unit or “chip.” One-chip cameras use a single computer chip to process the colors the camera sees, whereas with a three-chip camera, each chip processes a separate primary color, namely, red, green, or blue. One-chip cameras are satisfactory for most applications, but three-chip cameras provide superior optical clarity and color reproduction with images of photographic or broadcast quality. Alterations of the focus and the field of vision size are usually controlled by rings located on the camera head; more modern cameras allow the operator control of multiple functions such as white balance, other menu alterations, and image and video recording via buttons also located on the camera head. During laparoscopy, a mechanical insufflator regulates the flow of gas (usually carbon dioxide [CO2]) into the abdomen during creation of a pneumoperitoneum. A pneumoperitoneum allows the working space necessary to manipulate instruments and organs during laparoscopic procedures. During thoracoscopy, working space is created by formation of a pneumothorax, which occurs when air passes into the thoracic cavity through thoracic cannulas. The rigidity of the ribs prevents collapse of the thoracic wall during thoracoscopy, and so in most cases, no insufflation is required. Open cannulas, which allow air to pass into and out of the thoracic cavity freely, can be used for thoracoscopy. Insufflation can be used to increase the working space during thoracoscopy but is less well tolerated than a pneumoperitoneum; even 3 mm Hg pressure in the thoracic cavity causes significant cardiorespiratory depression.13 Insufflation is therefore used infrequently for thoracoscopic interventions. Modern insufflators allow monitoring of pressure within the abdominal cavity and prevent pressure rises above preset levels. In dogs, intraabdominal pressure of 15 mm Hg is considered to result in physiologically acceptable levels of cardiorespiratory depression, while providing more than adequate working space in most patients.15 Intraabdominal pressures of 8 to 10 mm Hg generally provide adequate working space in most dogs and cats. Insufflation hosing transmits the gas from the insufflator unit to the body cavity via the cannula. This hose should contain an inline microporous filter to prevent particulate matter or bacteria from passing from the gas cylinder into the patient, or conversely to prevent bodily fluids from moving in the opposite direction and contaminating the insufflator. Retrograde flow can occur when the CO2 canister is depleted intraoperatively, and pressure in the body cavity drives fluid retrograde along the hose.16 Movement of fluid in this manner can ultimately result in insufflator damage and cross-contamination of the next patient in whom the unit is used. Carbon dioxide is the most widely used insufflation gas in veterinary medicine. It does not support combustion as nitrous oxide (N2O) does, and it can therefore be used with various forms of electrocautery. It is cheap and colorless and is rapidly excreted from the circulation. Because of its high solubility, it is unlikely to form gas emboli. However, evidence now suggests that CO2 has detrimental effects on cellular, hormonal, and immunologic functions; this has led some surgeons to investigate the use of helium and other gases for insufflation.33 It is hypothesized that helium causes fewer changes in cardiorespiratory and immunologic status when compared with CO2.33 Standard fluoroscopy is acceptable for most of the more common respiratory, urinary, and gastrointestinal procedures; however, digital subtraction angiography is recommended for vascular procedures, particularly when performed in small caliber vessels with overlying structures such as bone and gas-filled intestine. Digital subtraction angiography is a computer software processing program that permits taking an initial noncontrast fluoroscopic image (the “mask”) and subtracting it from every subsequent image during a “run,” or series of recorded images. This permits improved vascular imaging and resolution without overlying structures obscuring the view, and allows the operator to access smaller structures more reliably without excessive amounts of contrast material (Figure 24-3). “Roadmapping” capabilities on some systems permit saving these contrast studies and placing them over real-time fluoroscopy images to obtain an actual map for guide wire, catheter, embolic, or stent manipulations. Principles of Abdominal Access: Veress Needle Technique A Veress needle is a specialized instrument used for creation of a pneumoperitoneum or pneumothorax (Figure 24-4). It consists of a sharp-tipped needle component that houses within it a blunt-tipped obturator loaded on a spring-like mechanism.46 The sharp component of the needle will penetrate the body wall, whose resistance will force the blunt obturator to retract into the shaft. Upon penetration of the body wall, however, resistance is lost, allowing the blunt-tipped obturator to spring forward, thereby shielding the abdominal viscera from injury. The major difference between access to the thoracic and peritoneal cavities is that with thoracoscopy, there is no need for insufflation because the ribs form a rigid frame that maintains a working space for the surgeon. For this reason, there is also no need to maintain a tight seal around cannulas that are placed into the thoracic cavity. Access into the thoracic cavity can be initiated with a Veress needle to allow a pneumothorax to develop, or a cannula without a diaphragm can be placed directly into the thoracic space; this allows air to enter when the trocar penetrates. Once the first cannula has been positioned, the telescope enters the chest, and additional instrument ports can be placed as needed under visual guidance. Some procedures can be performed just by using the visual access created by formation of a pneumothorax. When more advanced interventions are performed that require viewing of certain areas within the thoracic cavity, other techniques may have to be employed, such as one-lung ventilation or thoracic insufflation. Thoracic insufflation is not well tolerated even at low intrathoracic pressures.13 One-lung ventilation can be achieved in a variety of ways, including the use of bronchial blockers, selective intubation, or double-lumen endobronchial tubes. A large variety of sizes and designs of trocars and cannulas are available (Figure 24-5). Choices to be considered include single-use disposable versus resterilized nondisposable cannulas, blunt versus sharp trocars, and trocar-cannula assemblies versus trocar-less cannulas. Single-use disposable cannulas are generally made of lightweight plastic and are unlikely to slide out of port incisions—a common occurrence in small dogs and cats.
Equipment for Minimally Invasive Surgery
Laparoscopy and Thoracoscopy
Interventional Radiology/Interventional Endoscopy
Equipment
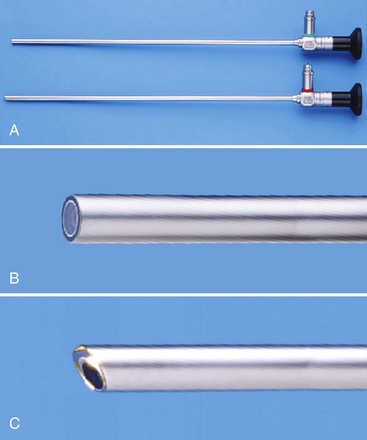
Imaging: Rigid Telescopes
Imaging: Cameras
Imaging: Tower Components
The Insufflator
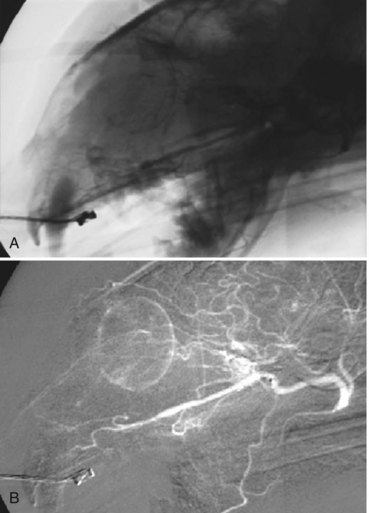
Imaging: Fluoroscopy
Laparoscopy/Thoracoscopy Instrumentation and Techniques
Principles of Thoracic Access
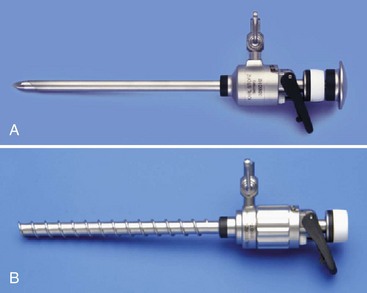
Trocars and Cannulas
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree