Chapter 8 Equine Recurrent Uveitis
Equine recurrent uveitis (ERU), also known as moon blindness, iridocyclitis, and periodic ophthalmia, has often been cited as the most common cause of blindness in horses.1–6 This immune-mediated, panuveitis syndrome has a reported prevalence of 2% to 25% in horses in the United States.2,5,6 However, field observations suggest that 1% to 2% of American horses suffer clinical disease serious enough to threaten vision.3 Fortunately, recent advances in the treatment of horses with ERU have led to an improvement in management of this disease. This chapter discusses some important facts about ERU, its causes, and treatment options for the affected horse.
ERU is characterized by recurrent episodes of intraocular inflammation separated, in most horses, by periods of remission (“quiescence”) in which there are no signs of active intraocular inflammation. These “bouts” of inflammation are separated by weeks to sometimes years. ERU generally develops after an initial bout of primary uveitis,3,5–9 but not every case of initial equine uveitis will develop into ERU (see later in diagnosis). Cause of the primary uveitis, environmental factors, and genetic makeup of the horse all play a role in the development of ERU, and each horse that develops signs of uveitis is considered at risk for ERU until several years have passed without relapse after the primary uveitis.
ERU-like diseases have been described in horses since Vegetius wrote of recurrent eye inflammation in the 4th century ad.1 Initially the syndrome was thought to be caused by the changes in the moon, hence the descriptive term moon blindness, still prevalent today. Much speculation occurred in the 18th, 19th, and early 20th centuries as to the cause(s) of ERU. Before 1940, prevailing theories included various infectious causes, hereditary predisposition, thyroid deficiency, riboflavin deficiency, climate, toxin hypersensitivity, and parasites.1,10,11 Early reviews describing the clinical examination and pathologic findings of ERU1,10,11 speculated further on infectious causes, and subsequent research was directed toward investigating bacterial etiologies. In 1947, Rimpau12 presented evidence connecting some cases of the syndrome with leptospirosis, and in the following year, Heusser published data linking positive serum agglutinin titers to L. interrogans serogroups Grippotyphosa, Pomona, and Australis in German horses.13 Although prevalence has varied with the geographic region, leptospirosis has been linked to initiation of spontaneous ERU around the world.3,14–28
Although leptospirosis has been linked to the initiation of ERU in many horses, other bacterial, viral, protozoan, and parasitic as well as noninfectious etiologies have been associated with the syndrome.2,29–36 The pathophysiology of ERU is far more complex than a simple systemic infection or traumatic episode. Research in horses, humans, and laboratory and domestic animals has shown that recurrent intraocular inflammation is multifactorial in origin, related to the genetic makeup of the individual and strongly immune mediated. Numerous investigations have described the variety of changes that accompany the syndrome in the anterior and posterior segment at the cellular level.7,37–45 Studies probing immune mechanisms responsible for triggering inflammatory episodes and tissue destruction, and relationships between the equine major histocompatibility complex (MHC) and ERU susceptibility are ongoing.7,38,46 ERU is an intricate disease complex, and defining the pathogenesis and risk factors will continue to challenge scientists and clinicians as research continues for effective therapies.
Clinical Anatomy and Physiology
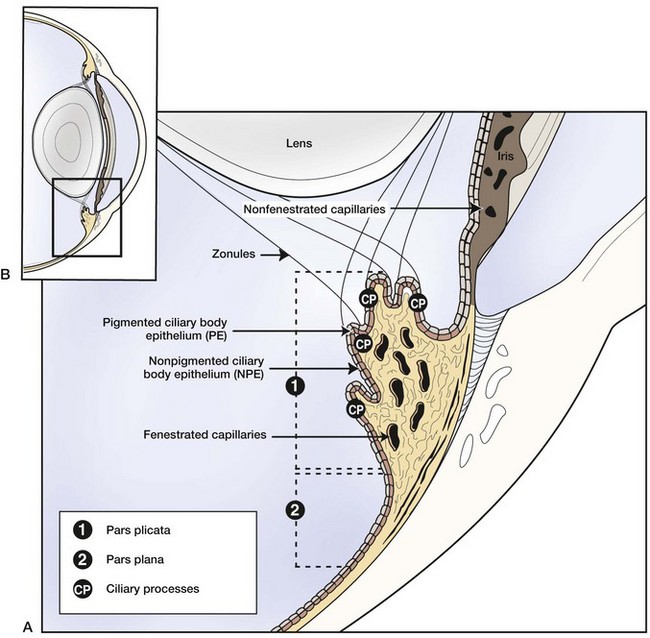
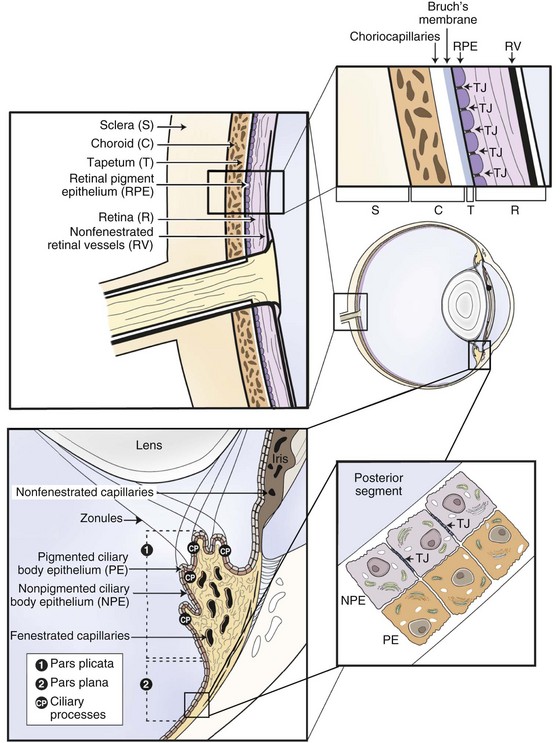
Equine recurrent uveitis is a syndrome that involves all aspects of the equine eye, so technically it would be considered a panophthalmitis, especially when most severe. However, the origin of the inflammation and the majority of the initial pathology are centered in the uveal tract, thus it is considered a panuveitis (Fig. 8-1). The uveal tract consists of the iris and ciliary body (the anterior uvea) and the choroid (posterior uvea).
Anatomy and Physiology of the Uveal Tract
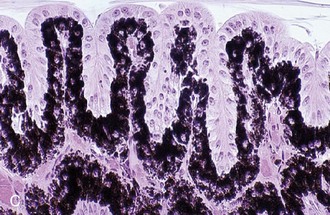
The two components of the anterior uvea, the iris and the ciliary body, contain heavily pigmented connective, vascular, and muscle tissue. The iris functions as a shutter that responds to prevailing light conditions, and the ciliary body produces aqueous humor through active secretion and ultrafiltration of plasma. Both the iris and ciliary body have a large number of blood vessels within their connective tissue, and the inner aspect of both structures is lined by a double layer of epithelium, which has an important role in the pathophysiology of ERU. The layer of epithelium closest to the connective tissue is pigmented, and the boundary layer closest to the vitreous is nonpigmented. The clinical anatomy and microanatomy of the anterior portion of the uvea are described in Chapter 6, Diseases of the Uvea.
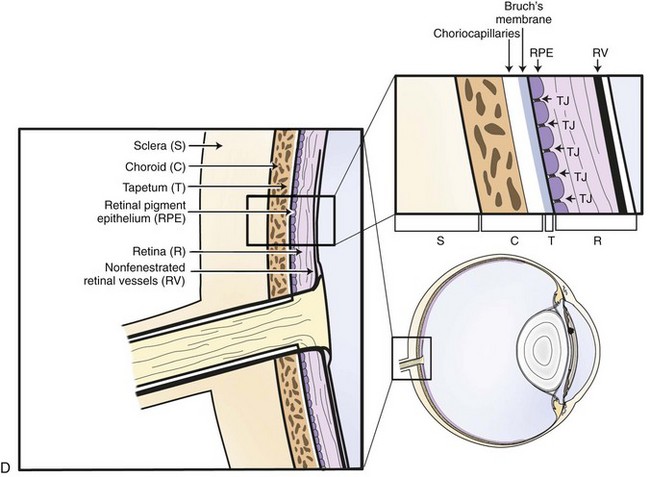
The choroid, or posterior part of the uvea, functions as the primary vascular supply of the horse retina (see Chapter 10). It lies between the sclera and the retina and contains the tapetum, the fibrous reflective layer (See Chapter 10 for a complete description of the choroid).
The uveal tract contains most of the blood supply of the eye and is in direct contact with peripheral vasculature. Therefore, diseases of the systemic circulation (e.g., septicemia, bacteremia, etc.) will also affect the uveal blood circulation. There is a barrier between this blood circulation and the internal aspects of the eye, termed the blood-ocular barrier (Fig. 8-2). The blood-ocular barrier consists of the blood-aqueous barrier (i.e., tight junctions between the nonpigmented epithelial cells of the ciliary body and nonfenestrated iridal blood vessels) and the blood-retinal barrier (i.e., tight junctions between the cells of the retinal pigmented epithelium and nonfenestrated retinal vessels). These semipermeable barriers normally prevent large molecules and cells from entering the eye and help the intraocular fluids remain clear. The blood-ocular barrier also limits the immune response to the internal aspects of the eye, causing the eye to be considered an immune-privileged site.47 With trauma or inflammation, these barriers can be disrupted, allowing blood products and cells to enter the eye. Flare, cell accumulation, or haze in the aqueous or vitreous are clinically observable signs of the disruption of the blood-ocular barrier that occurs in ERU. Disruption of the barrier enables activation of various host immune responses, including antibody production to self-antigens that are not normally recognized by the horse’s own immune system, as well as antibody production to foreign antigens inside the eye.
Physiology of Equine Recurrent Uveitis
The uvea and aqueous humor nourish a number of anatomically and functionally dissimilar components in the eye. Inflammation in uveitis is thus associated with inflammation and/or dysfunction in, variably, the cornea, sclera, lens, retina, and optic nerve. The physiology of inflammation in the non-uveal parts of the eye is dependent on the anatomy and physiology of each affected area (see Chapters 6, 7, and 10).
Transparency of the cornea is compromised when inflammatory mediators in the aqueous humor cause altered function of the corneal endothelium. Disruption of the endothelial sodium/potassium–adenosine triphosphatase (Na+/K+-ATPase) pump that normally keeps the cornea relatively dehydrated contributes to opacity changes. Resultant corneal stromal edema causes focal or diffuse opacity and general “steaminess” of the cornea. Edema can become permanent if endothelial dysfunction is severe. Corneal cellular infiltrate or extensive vascularization is not a primary feature of ERU; if present, the clinician should rule out primary corneal disease such as stromal abscessation or immune-mediated keratitis.48,49
Impact of Equine Recurrent Uveitis on the Horse Industry
Ocular diseases are among the most common health disorders of horses. In fact, in the 2005 U.S. Horse Council report,50 in the study’s 12 months, 6.5% of farms in the United States had at least one horse with an eye problem, and ocular disease was the fifth most common equine disorder. Of these eye diseases, inflammation of the eye, such as keratitis and ERU, are the most common causes of blindness.50 Prevalence of infectious ocular disease and immune-mediated ocular disease in horses is higher than in any other animal other than human beings. In humans, these inflammatory ocular diseases are characterized by molecular features, and many are known to be hereditary or associated with genetic phenotypes. This is similar to horses, but much more knowledge is needed regarding genetic inheritance and ERU susceptibility. Because ERU has a high prevalence rate across horse breeds in the United States, the economic impact of this disease on the equine industry could be very high, especially when one factors in the effect of ERU on disruption of training, decreased performance, and disqualification of horses from competition (due to medication use, etc.; see more information in Chapter 2). Horses with ERU have decreased value as a result of vision loss, and many horses that are blinded by ERU must be euthanized for practical and economic reasons. Treatment, veterinary care, and personnel costs add to the economic impact of the disease.
Classification of Equine Uveitis
Equine uveitis is characterized as primary or recurrent. Recurrent uveitis is further characterized into three main clinical syndromes in horses: classic ERU, insidious ERU, and posterior ERU (Table 8-1). Because ERU most commonly involves all areas of the uveal tract (i.e., panuveitis), the human anatomic classification of uveitis (i.e., anterior, intermediate, and posterior uveitis) is not particularly distinguishing in horses.
Table 8-1 | Clinical Classification of Equine Uveitis
| CLASSIFICATION | DESCRIPTION | |
|---|---|---|
| PRIMARY UVEITIS | First or persistent bout of ocular inflammation (see Chapter 6 for causes) | |
| SYNDROME OF EQUINE RECURRENT UVEITIS (ERU) | ||
| Clinical classification | Classic recurrent | Active inflammatory episodes followed by periods of minimal ocular inflammation |
| Insidious | Persistent low-grade intraocular inflammation without overt signs of discomfort | |
| Posterior | Recurrent inflammation primarily in the vitreous, retina, and choroid | |
| Stage of chronicity | Active/acute | Actively inflamed eye with signs of intraocular inflammation |
| Quiescent | No clinical evidence of active internal inflammation and a comfortable eye | |
| End-stage | Blind eyes with phthisis bulbi with possibly dense cataract, luxated lens, detached retina, and/or loss of normal pupillary architecture | |
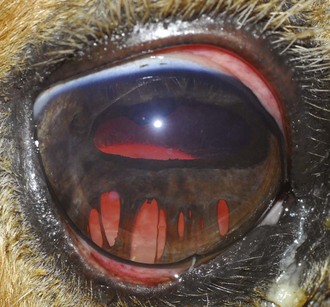
Classic ERU is most common and is characterized by active inflammatory episodes in the eye followed by periods of minimal observable ocular inflammation. The acute active phase of ERU predominantly involves inflammation of the iris, ciliary body, and choroid, with concurrent involvement of the cornea, anterior chamber, lens, retina, and vitreous (Fig. 8-3). After variable periods of time, the quiescent phase is generally followed by further and increasingly severe attacks of uveitis. In many horses, the repeated episodes of inflammation cause development of cataract, intraocular adhesions, phthisis bulbi, and vision loss (Fig. 8-4).5,10,29,36,51
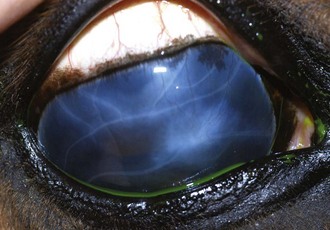
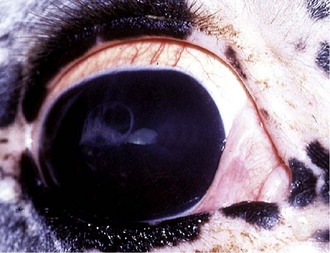
Insidious ERU is characterized by a low-grade intraocular inflammation that does not manifest as outwardly painful episodes but has a gradual and cumulative destructive effect, which leads to degeneration of ocular structures and chronic clinical signs of ERU (Fig. 8-5). This type of ERU is most commonly seen in Appaloosa and Draft breed horses.
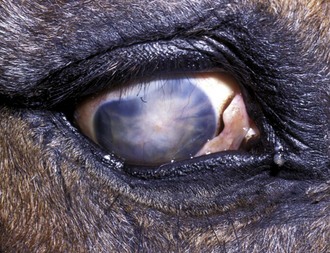
Posterior uveitis is inflammation predominantly in the vitreous, retina, and choroid (although mild anterior segment inflammation is commonly present). Clinical signs include bouts of vitreal inflammation, cloudiness, retinal detachment, and vision loss (Fig. 8-6). Chronically, these horses may develop cataracts, retinal detachments, vitreal degeneration (with or without fibrous strands), and retinal degeneration. This syndrome is most common in Warmbloods, Draft breeds, and European horses.
ERU may be further separated according to stage of chronicity, with cases labeled as “active or acute,” “quiescent,” or “end-stage” (see Table 8-1). Acute or active cases show active pain and observable internal inflammation manifested as blepharospasm, mild to moderate corneal edema, aqueous flare, aqueous cells, hypopyon, iris hyperemia, miosis, hypotony, vitreal cellular infiltrate, and possibly retinal inflammation and detachment (Fig. 8-7). Quiescent cases are outwardly comfortable and show little active acute internal inflammation on clinical examination. However, quiescent cases may have chronic inflammatory sequelae such as synechiae or cataracts. End-stage cases are usually eyes with chronic ERU with severe and often blinding changes such as phthisis bulbi, dense cataract, luxated lens, detached retina, and/or loss of normal pupillary architecture (Fig. 8-8).
Clinical Appearance of Equine Recurrent Uveitis
No gender predilection has been reported for ERU. Age of the initial episode is variable. One study of 160 horses with ERU found that half presented before 12 years of age, a time when horses are in their prime performance years.3 Clinical signs are variable and depend on the stage of the episode, preexisting chronic ocular changes, the nature of the inflammation, and the anatomic location of the majority of the inflammation.
Ocular Signs: Anterior Segment
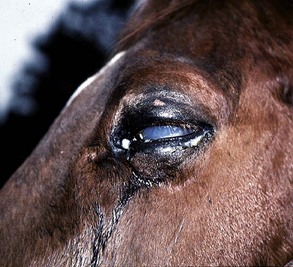
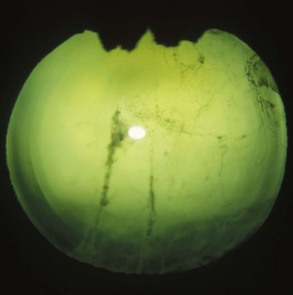
A horse experiencing a classic acute inflammatory episode presents with pain, lacrimation, and blepharospasm (see Fig. 8-7). The severity of the clinical signs varies from a slightly closed eye to a horse that will not tolerate any manipulation of the periocular structures without sedation. Severe blepharoedema may make ocular examination difficult. The cornea may be edematous, with the resultant opacity being most marked at the periphery. Cellular infiltrate (i.e., white or yellow corneal infiltrate) or extensive corneal vascularization is not common in ERU, and their presence may suggest primary corneal disease instead of ERU. Short, deep, circumlimbal vascularization is frequent, however, and the length of the vessels are proportional to the duration of the episode. Fluorescein dye uptake will be negative, unless the horse has suffered a secondary ulcer from self-trauma. The anterior chamber may appear cloudy or hazy due to aqueous flare, and hypopyon or hyphema may be present in the ventral anterior chamber. Keratic precipitates may be visible as focal white spots on the endothelium of the cornea. A cardinal sign of active uveitis is miosis (see Fig. 8-3),36 always present in an acute episode unless mydriatics have been administered or synechiae distort the pupil. The iris may be dull in color, show mottled pigmentation or depigmentation, or have changes in color or fibrosis (Fig. 8-9). Corpora nigra degeneration with thinning and depigmentation of the iris margin is very characteristic of ERU and may develop after several inflammatory episodes (see Figs. 8-4, 8-5, 8-8, and 8-9).
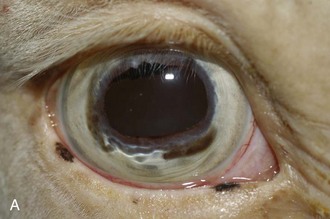
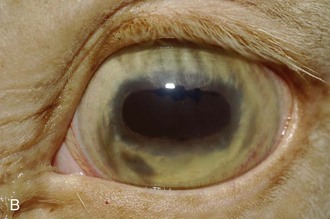
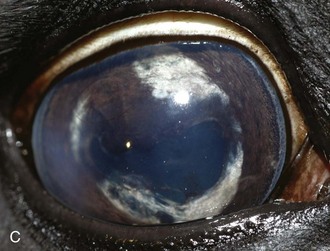
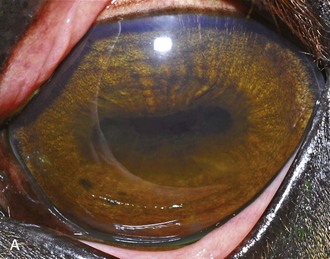
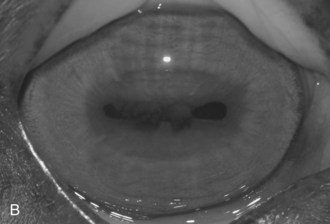
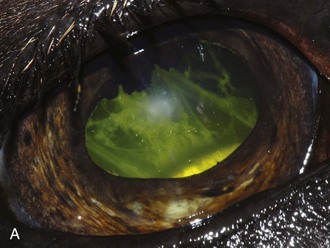
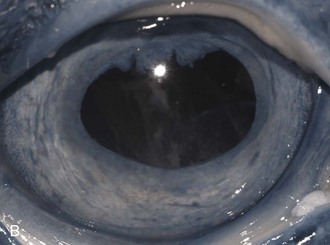
Figure 8-9 Iris color changes in equine recurrent uveitis. In acute uveitis, the iris can change to a yellow or even green color as a result of infiltration or inflammatory cells and serum. This color change is most prominent in blue or light-colored irises. A, Noninflamed right eye of a light-colored horse. B, Left eye of the same horse with a discolored iris due to active uveitis. Note peripheral corneal vascularization and miosis. C, Depigmentation of a dark iris also occurs but much less commonly than hyperpigmentation (see Fig. 8-8). This horse has progressive iris depigmentation associated with recurrent uveitis. Note other signs of chronic inflammation, such as corpora nigra atrophy, dyscoria, and synechia.
A horse with insidious ERU does not appear painful, but examination of the eyes often shows inflammatory changes suggestive of chronic inflammation. Commonly the conjunctiva and episclera are hyperemic and mild to moderate blepharitis is often observed (see Fig. 8-5). The cornea may be mildly edematous, dull, or hazy. Corneal cellular infiltrate or extensive vascularization is not a feature of insidious ERU, although focal limbal vascularization may be discernible. Fluorescein dye uptake will usually be negative. Aqueous flare (1 to 2+) is usually visible on slit-lamp biomicroscopy. The iris is discolored and hyperpigmented. Degeneration of the corpora nigrans is a hallmark clinical sign of insidious ERU and is commonly associated with iris atrophy and fibrosis (see Fig. 8-5). The pupil is usually miotic with a sluggish pupillary light reflex.
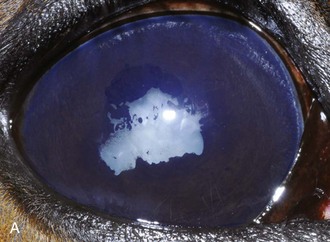
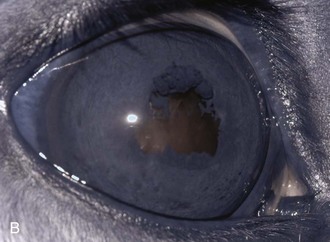
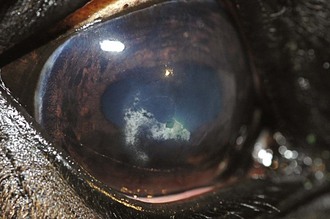
Inflammatory sequelae and scarring are often apparent in the anterior segment of both classic and insidious ERU cases. If the horse has had previous corneal disease (e.g., corneal ulcer), corneal scars may be present. Calcific band keratopathy is occasionally seen in the corneal subepithelium or outer stroma in horses with chronic ERU (Fig. 8-10). Posterior synechia, pigment on the anterior lens capsule, or pupillary occlusion are common manifestations of past damage in both classic and insidious ERU (Fig. 8-11). Focal or diffuse cataracts may be apparent (Fig. 8-12). Dense cataracts may obscure visualization of the posterior segment, and lens luxation or subluxation are observed occasionally (Fig. 8-13).
Most eyes with acute uveitis are hypotensive, with intraocular pressures (IOPs) of 5 to 12 mm Hg, and commonly the anterior chamber is shallow. Glaucoma is a common sequela to all types of ERU, and this secondary glaucoma may be the most common underlying cause of glaucoma in horses (see Chapter 9). Horses that develop secondary glaucoma will have diffuse corneal edema that does not respond to antiinflammatory medications, or at least does not respond as well as observed in previous bouts. IOPs typically range from 35 to 80 mm Hg in these eyes. Enlarged eyes and development of cornea striae secondary to glaucoma occur with chronic ERU (Fig. 8-14).
Ocular Signs: Posterior Segment
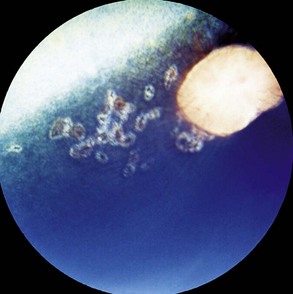
Some horses have no observable vitritis but show chorioretinal scarring of the peripapillary region or other evidence of retinal degeneration. The most common patterns of chorioretinal scarring are seen in the nontapetal area near the disc and present as either multiple, small, circular focal areas of depigmentation with a central area of hyperpigmentation (“bullet-hole” scarring) or wing-shaped areas of hypopigmentation nasal and temporal to the optic disc (“butterfly” lesions [Figs. 8-15 and 8-16]).
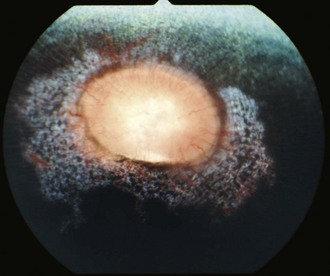
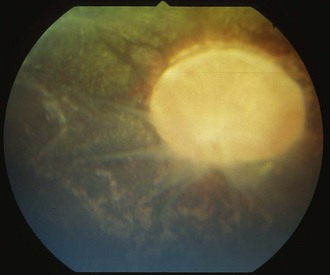
Other horses demonstrate severe vitritis where detail of the fundus is obscured and the optic disc appears orange-red owing to the vitreal inflammation and haze. Liquefaction of the vitreous is common, as are visible strands of clumped infiltrating mononuclear cells and inflammatory products (floaters [Fig. 8-17]). These opacities appear to float and move easily in the vitreous in response to eye movements. Fibrinous traction bands may be apparent as white spike-like structures that radiate from the perimeter of the optic disc (Fig. 8-18). Retinal detachments may be present and appear as a veil-like transparency that obscures fundic detail. In fact, ERU is one of the most common causes of retinal detachment in horses.52
Chronic End-Stage Equine Recurrent Uveitis
Horses with chronic end-stage ERU show variable degrees of discomfort and inflammatory sequelae. Recurrent episodes of inflammation may subside but may be replaced by constant low-grade discomfort from blepharitis, mucopurulent lacrimation, conjunctivitis, and ocular irritation (Fig. 8-19). Some horses appear comfortable and do not have signs of recurrent episodes of inflammation. Ocular structures often become severely scarred if eyes undergo phthisis bulbi. In these eyes, corneal scarring may be dense and develop folding and anterior synechiae (Fig. 8-20). Iris architecture may be lost or indistinct. Cataracts and lens luxations are common, and the color of the lens may become yellow. Complete retinal detachment is also common. The nictitans may become elevated or prolapse as the globe becomes phthisical, and the mucosa may show chronic inflammation. A head tilt or “star-gazing” head posture is sometimes observed in blind horses with end-stage ERU (Fig. 8-21).
Bilateral Versus Unilateral Ocular Involvement
One study found that approximately 50% of eyes with leptospiral-induced ERU were bilateral.53 In this same study, 81% of Appaloosa horses with ERU had bilateral involvement.53 Of a small series of 32 horses that were seronegative to L. interrogans serogroup Pomona and were of non-Appaloosa breeding, 38% had bilateral involvement (Table 8-2).53 In a recent study reviewing 186 cases of ERU that received a cyclosporine implant, only 16% had bilateral disease.54
Immunologic Aspects of Equine Recurrent Uveitis
Pathogenesis of ERU is immune mediated. Although the etiology of ERU is still under discussion, there is agreement that a dysregulated immune response causes the disease.7 It explains the recurrences of inflammation, the positive effect of corticosteroids (or other more selective immunosuppressive agents), and the insufficient therapeutic success of antibiotics. The relevance of the inflammatory reaction is underscored by the successful therapeutic strategy of immunosuppression, the gold standard therapy to control ERU for decades.55 Use of corticosteroids is the therapy of choice, but a more precise local targeting of T cells only has been shown to be effective in controlling recurrent disease by using an intraocular cyclosporine device.54–58
General Features of Ocular Immunology and Equine Recurrent Uveitis
Physiologically, the inner eye is devoid of immune cells, and the eye maintains an immunosuppressive environment through, for example, factors expressed in the vitreous such as transforming growth factor β (TGF-β). The eye has long been recognized as an immune-privileged organ. Immune privilege in the eye was originally ascribed to its separation from the systemic immune system by the blood-ocular barrier, lack of lymphatics, and the presence of limited numbers of resident leukocytes. But extensive detailed work has demonstrated that privilege is in fact a far more active process than this. The blood-ocular barrier is a specialized endothelium with tight junctions that control cell traffic in a highly regulated fashion. Naïve T cells cannot cross the normal blood-retinal barrier because of the high shear stress in the retinal vessels and the lack of appropriate adhesion molecules. Expression of chemokines such as RANTES in the ciliary epithelium may play a role in recruitment and activation of leukocytes in diseased eyes.41 Furthermore, inner and outer blood-retinal barriers keep cells from the healthy inner eye.59 Retinal blood vessels that are similar to cerebral blood vessels maintain the inner blood-ocular barrier. This physiologic barrier comprises a single layer of nonfenestrated endothelial cells which have tight junctions. The retinal pigment epithelium maintains the outer blood-retinal barrier.59 Since the horse retina is mainly avascular, the outer blood-retinal barrier (retinal pigment epithelium) mainly keeps the immune effector cell-free environment in equine eyes.60
In ERU, considerable amounts of leukocytes are able to enter the inner eye and cause damage to the inner ocular tissues.38 Analysis of the infiltrating cells revealed that the majority of cells are lymphocytes in most ERU cases.38 These cells are predominantly CD4-positive T cells that secrete proinflammatory cytokines such as interleukin 2 (IL-2) and interferon γ (IFN-γ).40 The IFN-γ-producing phenotype is named TH1 helper cell. TH cells are involved in activating and directing other immune cells and are particularly important in the immune system. They are essential in determining B-cell antibody class switching, in the activation and growth of cytotoxic T cells, and in maximizing activity of macrophages.61 It is this diversity in function and their role in influencing other cells that gives helper T cells their name. Until recently, this cell type of IFN-γ-producing TH1 effector cells was considered the main pathogenic pathway in autoimmune diseases. TH17 cells are a newly identified subset of CD4+ T-helper cells producing IL-17.62 They are found at interfaces between the external environment and the internal environment, such as the skin and lining of the GI tract. Numerous immune regulatory functions have been reported for the IL-17 family of cytokines, presumably due to their induction of many immune signaling molecules. Most notably, IL-17 is involved in inducing and mediating proinflammatory responses.62 IL-17 induces the production of many other cytokines, chemokines, and prostaglandins from many cell types. The increased expression of chemokines attracts other cells, including neutrophils but not eosinophils. TH17 lymphocytes are implicated in a variety of immune-related diseases, including rheumatoid arthritis.63 Recently, TH17 and IL-17 have been found to increase in patients with juvenile idiopathic arthritis (JIA) and Behcet’s disease, each of which may be associated with uveitis.64 Novel data from experimental uveitis models in rodents also point to a significant role of IL-17 in uveitis.65,66 It is interesting to note that whereas the TH17 line typically recruited a mostly granulocytic inflammatory infiltrate into the eye, the TH1 line recruited a predominantly mononuclear infiltrate. Notably, the severity of tissue pathology induced by TH1 cells was no less than that induced by TH17 cells.67 To date, it is not known if a certain percentage of ERU cases are caused through TH17 cells, because adequate tools to address this question are lacking at the moment. Interestingly, a few ERU cases had a pure granulocytic infiltrate in the inner eye.39 This was also seen in an experimental model of induced uveitis in horses using interphotoreceptor retinoid-binding protein (IRBP) as an autoantigen. Inflammatory cells differed between experimental horses.68 Differences in the immunologic response profile could account for pathologic and clinical disease manifestation differences, but this is speculative to date.
Autoantigens and Equine Recurrent Uveitis
Uveitis is a clinically heterogeneous disease. Although the antigenic triggers of autoimmune uveitis are still under discussion, there is a large body of evidence implicating responses to retinal antigens in the etiology and/or progression of the disease. One of the explanations that has been offered for the clinical heterogeneity of uveitis cases is a difference in the antigens being recognized. Many, though certainly not all, ERU cases have detectable immunologic responses to retinal antigens, most often to IRBP.39 IRBP is a large glycoprotein (140 kD) known to bind retinoids and found primarily in the interphotoreceptor matrix of the retina between the retinal pigment epithelium and the photoreceptor cells. It is thought to transport retinoids between the retinal pigment epithelium and the photoreceptors, a critical role in the visual process. Further, immune reactions of diseased horses are additionally directed against S-antigen (S-Ag),69,70 a photoreceptor protein found in rods and in the pineal gland, exerting an inhibitory function in the light transduction cascade. In almost all ERU cases, serum and vitreal autoantibodies against both autoantigens are detectable. Since antiretinal antibodies do not seem to be essential for the induction of uveitis, as shown in several EAU models,71 and the predominating infiltrates in eyes of ERU horses are T-helper cells, the T cell specificity is even more interesting. Vitrectomy, as a therapeutic procedure, allows one to obtain sufficient numbers of vitreous infiltrating cells from diseased horses for immunologic studies and compare the reactions of peripheral and intraocular lymphocytes.
Whereas peripheral blood-derived lymphocytes (PBL) do not proliferate after in vitro stimulation with retinal antigens,39,72 cells from the eye strongly responded.39,72 In most cases, a clear response to one or several IRBP-derived peptides was observed. This is in accordance with other autoimmune diseases, where responses to autoantigens are also rarely seen with peripheral lymphocytes. Only in some patients or at certain time points will PBL respond to autoantigens.73 The low frequency of antigen-specific peripheral blood lymphocytes, even in advanced cases of uveitis, has been discussed as one reason for poor results in proliferation assays.
Further analysis of the immune response pattern of ERU cases revealed a novel yet unknown protein as a uveitis autoantigen. An immune response to cellular retinaldehyde-binding protein (CRALBP)74 was detectable in a large percentage of ERU cases. CRALBP was detected as a novel uveitis autoantigen. Screening the immune response of ERU cases to a map of retinal proteins, CRALBP could be verified as a novel ERU autoantigen74 and was subsequently tested with samples of human autoimmune uveitis patients.75 Fifty-four percent of tested patients were CRALBP autoantibody positive, thereby underscoring the relevance of ERU as a model for human autoimmune uveitis. Considerable evidence indicates that CRALBP is a component of the rod and cone visual cycles, the sequences of reactions responsible for the regeneration of 11-cis-retinal after photoisomerization. The protein is abundant in RPE cells, where many of the reactions of the rod visual cycle take place, and in Mueller cells, which have been implicated in cone visual pigment regeneration.
Earlier studies had shown that experimental uveitis induced in guinea pigs with soluble retinal proteins also led to involvement of the pineal gland in the inflammatory process.76 Pineal glands were infiltrated by lymphocytes and observable before retinal involvement.76 Interestingly, the same group later also demonstrated pinealitis in ERU cases.43,77,78 Septal areas of pineal glands from horses with uveitis had clusters of MHC class II antigen-expressing cells and T lymphocytes.77
Pathogenesis of Recurrent Disease
Current concepts to explain the origin and perpetuation of autoimmune diseases include molecular mimicry,79 bystander activation,80 and epitope spreading.81,82 These mechanisms do not exclude each other but could appear together and even interact. Epitope spreading is defined as the diversification of epitope specificity from the initial focused, dominant, epitope-specific immune response, directed against a self or foreign protein to cryptic epitopes on that protein (intramolecular spreading) or other proteins (intermolecular spreading).
The immune response consists of an initial magnification phase and a later downregulatory phase to return the immune system to homeostasis. In most autoimmune diseases, several autoantigens participate in the pathogenesis,83 and epitope spreading is accountable for disease induction, progression, and inflammatory relapses.84–88 The shifts in immunoreactivity could account for the remitting/relapsing character of ERU. Different target antigens may be important for a given individual, depending on the MHC background. Genetic background and antigens encountered influence the direction and extent of epitope reactivity and probably play an important role in the heterogeneous clinical manifestations of disease.89 In many autoimmune diseases, epitope spreading is suspected to occur as a result of an immune response against endogenous target antigens first and secondary to the release of self-antigen during the chronic autoimmune response.89 The formation of new antibodies may determine a different clinical picture. For example, the transformation of anterior uveitis to posterior uveitis may be an excellent example of the effects of epitope spreading. Through tissue damage, cryptic or hidden epitopes on the same molecule will be suddenly presented to the immune system. The end result is that every target antigen generally contains several epitopes, each of which reacts with a T cell or antibody response of different specificity and affinity. Thus, epitope spreading in autoimmune diseases results in the detection of an increasing array of autoantibodies against various target antigens. Studies characterizing the shifting immune response of ERU-diseased horses are currently underway, but initial studies have confirmed epitope spreading in a high percentage of cases.37
Analyses of autoantigen and epitope specificity of autoaggressive T cells from ERU cases already revealed evidence for inter- and intramolecular epitope spreading.37 Epitope spreading is only pathogenesis associated if the targeted epitope has pathogenic potential. Therefore, it was essential to evaluate the uveitogenic potential of all involved autoantigens directly in the horse. Surprisingly, S-antigen, which is seen as the major uveitis autoantigen in human autoimmune uveitis and is used by many researchers as autoantigen to experimentally induce uveitis in Lewis rats for uveitis research, widely failed to induce uveitis in horses.90 S-Ag-induced uveitis is monophasic in rats69 and was also monophasic in one horse that developed uveitis after S-Ag injection.90 In contrast, CRALBP and IRBP proved their uveitogenicity in the horse with an incidence of 100%.74 Further, both autoantigens were capable of reinducing uveitis in the horse,74 closely resembling the clinical course of spontaneous ERU. Equine experimental uveitis induced with CRALBP or IRBP is to our knowledge the only animal model where relapses of an autoimmune disease can be reinduced in a well-defined and predictable manner.
Uveitic relapses are notable even in phthisical and blind eyes.91 Histopathologic examination of ERU eyes and eyes from experimentally induced uveitides revealed an almost complete loss of the photoreceptor outer segments in advanced stages of disease.38,69 Since the photoreceptor outer segments host two major autoantigens, retinal S-Ag (arrestin) and IRBP,92 the ongoing immune pathology after destruction of the physiologic expression sites of these proteins was difficult to explain. In other autoimmune diseases, such as Hashimoto’s thyroiditis, a termination of autoimmune attacks is notable after depletion of autoantigens.93,94 We therefore examined autoantigen expression levels of IRBP, S-Ag, and CRALBP in uveitic retinas of several clinical stages in comparison to healthy control retinas. IRBP, S-Ag, and CRALBP were clearly detectable in proteomes of normal equine retina and in retinas with different stages of uveitis. Although the composition of retinal proteomes differed considerably between healthy and diseased state,95 IRBP, S-Ag, and CRALBP amounts remained unchanged.96 Substantial photoreceptor damage in uveitic retinas tested in this study was evident by measuring rhodopsin expression levels that were reduced to 27% of original expression. The unchanged total amount of three major retinal autoantigens indicates that the autoantigenic target protein is present despite considerable destruction of its hosting structures and can thus trigger unabated relapses in patients with blind eyes.
Differentially Regulated Proteins in Equine Recurrent Uveitis
Although retinal autoantigen–specific TH1 cells have been demonstrated to trigger disease progression and relapses, the molecular processes leading to retinal degeneration and consequently blindness remain as yet unknown. Systematic exploration of the intraocular proteomes of spontaneous uveitis and healthy controls has made it possible to identify several differentially regulated proteins that belong to pathways involved in immune response and the maintenance of the blood-retinal barrier.95,96
One upregulated candidate in the retina in ERU is, amongst others, complement component C3.97 Novel data indicate an activated complement system also in sera of ERU cases.97
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree