Chapter 1 Equine Ocular Examination
Routine and Advanced Diagnostic Techniques
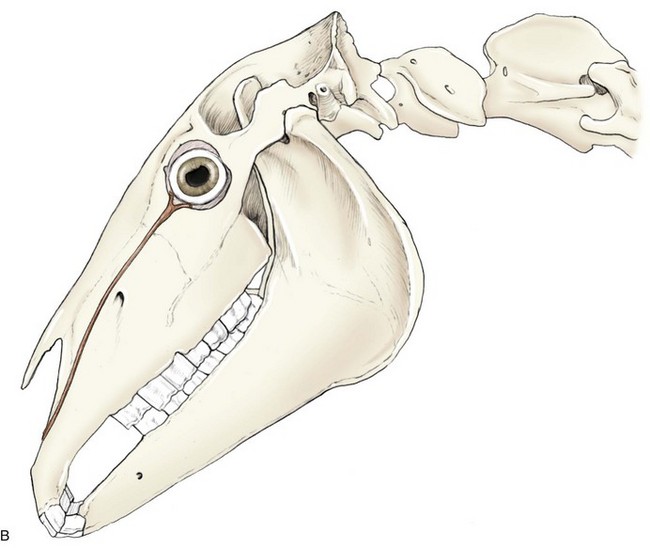
The basic and essential aspect of equine ophthalmology is a complete, thorough ocular examination. In this chapter, ophthalmic examination of the horse will be discussed, with emphasis on techniques, tools and instruments, and other diagnostic modalities. Understanding normal equine ocular anatomy is integral to interpreting the ocular examination. Anatomy of the equine eye (Fig. 1-1) is described in detail in subsequent chapters and will not be repeated here, although cross-references to relevant sections in the book will augment our discussion. Excellent reviews of equine eye and head anatomy can also be found in other sources.1–6
Both routine and advanced ophthalmic diagnostic techniques are described. Examination of the equine eye includes obtaining the history and signalment, inspecting the patient in a well-lit environment, examining the ocular structures in a darkened environment, facilitating the examination with restraint, sedation, and local nerve blocks, and collecting relevant diagnostic samples or data.4,7–10 Indications and technique for advanced diagnostics—ultrasound, electroretinography, computed tomography, and magnetic resonance imaging—will also be discussed in this chapter.
Medical History
A thorough medical history relevant to the ocular examination should include how the animal is used (e.g., pet or performance) and its living environment. Additional information that should be collected includes any history of travel, vaccination history, deworming schedule, and presence of concurrent or previous medical problems such as nasal discharge, presence of stridor, previous trauma to the head, and status of other horses on the premises with similar signs. Characterization of the primary complaint should include the onset and initial clinical signs, treatment given and response to that treatment, progression and duration of the ocular problem, and current therapy. Signalment can provide an important clue as to the cause of many ophthalmic conditions (e.g., congenital stationary night blindness in the Appaloosa, hereditary cataracts in the Morgan horse). Existing medical therapy can also greatly influence findings on ophthalmic examination. For example, a finding of mydriasis on the ophthalmic examination could be caused by use of topical atropine, which may result in mydriasis for up to 14 days in horses.11 Depending on the specific complaint, further information may be required, such as a thorough description of vision loss (e.g., light versus dark, moving objections, one eye or both, etc.).
Routine and Advanced Equipment Required for the Ophthalmic Examination
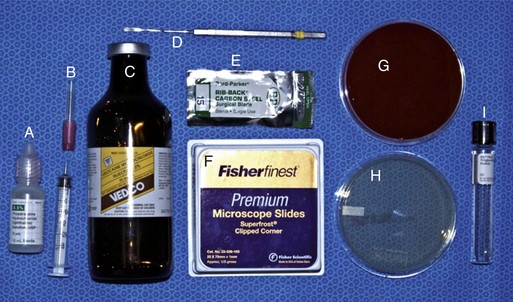
Prior to any examination, the proper equipment to perform the examination is needed. Although there are some differences in opinion and personal preferences among equine ophthalmologists, Box 1-1 lists the routine and advanced equipment a clinician should have available for the ophthalmic examination. Routine materials for the examination are shown in Figures 1-2 and 1-3. Indications and methods of use will be described in later sections of this chapter.
Box 1-1 | Equipment and Supplies for General Equine Ophthalmic Examination
Overview and Methods of the Equine Ocular Examination
The ocular examination in the horse, like any physical examination, should be performed in a systematic manner. The general order of steps to be taken in the examination is listed in Box 1-2. The initial examination of the equine eye should occur prior to sedation and should take place in a well-lit area. The examination area should be quiet, away from distractions, and if possible away from other horses. The menace response and other subjective vision testing (e.g., maze testing) and evaluation of the pupillary light reflex (PLR) should be performed before sedation. For accurate evaluation of PLRs, a bright focal light source and a darkened examination area are often required. To adequately examine the cornea and internal structures of the eye, the horse must be examined in a darkened stall or in stocks in a room where the lights can be dimmed. Ideally, stocks are preferred because they will better protect the examiner from accidental (or purposeful!) movements of the horse, which can be exaggerated and unpredictable when the horse is tranquilized.
Box 1-2 | General Order of Steps in the Routine Equine Ocular Examination
Initial Examination
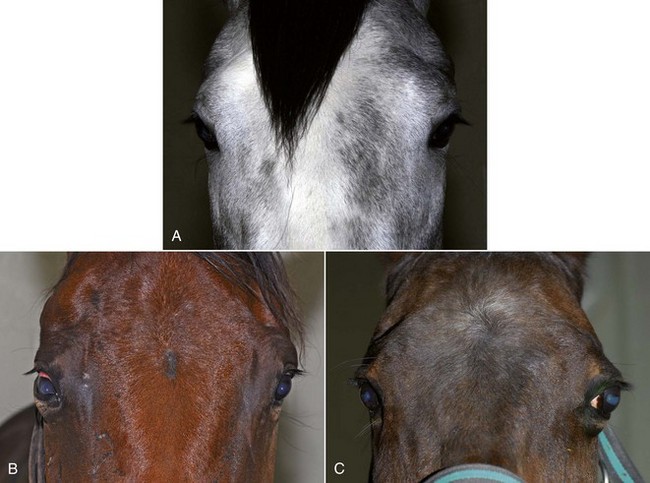
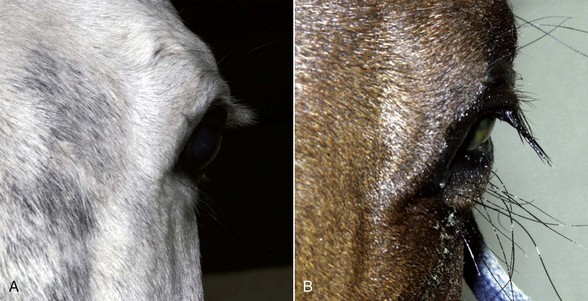
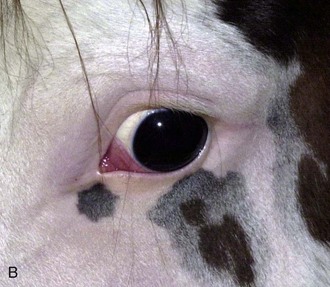
With the examiner positioned in front of the horse, the head, bony orbits, eyelids, globes, and pupils should be examined for symmetry (Fig. 1-4) before touching the eyelids, giving sedation, or use of eyelid nerve blocks. Ocular comfort may be assessed by evaluation of palpebral fissure size and symmetry, position of the eyelashes, ocular discharge, and blink rate.4,8,9 The upper eyelashes of the healthy horse are nearly perpendicular to the cornea (Fig. 1-5, A).8 A ventral or downward direction of the eyelashes in relation to the cornea may indicate blepharospasm, enophthalmos, or ptosis (see Fig. 1-4, B), while an upward deviation may indicate exophthalmos or an enlarged eye (see Fig. 1-4, C).8
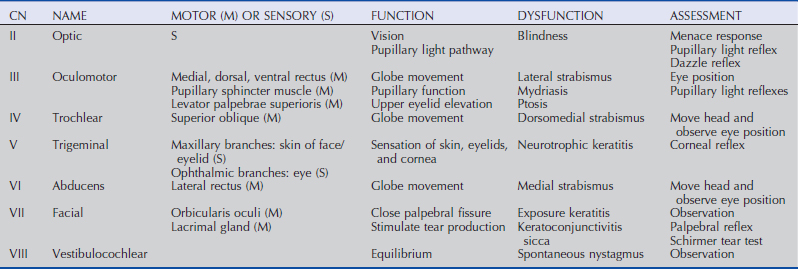
A cranial nerve evaluation (specifically, cranial nerves II, III, IV, V, VI, VII) is then performed before any sedation is induced. These cranial nerves are assessed via the menace response, pupillary light and dazzle reflexes, globe and eyelid position and mobility, and sensation of ocular and adnexal structures.8,9 Examination of the cranial nerves is discussed in more detail in the following section.
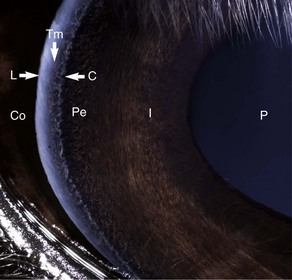
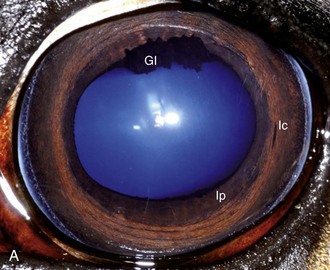
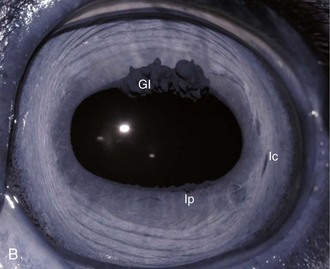
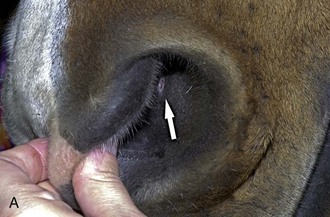
The cornea should be examined for abnormalities (e.g., opacities, ulceration, blood vessels, edema) by using transillumination and/or slit-lamp biomicroscopy. Evaluation of resting pupil size, shape, and mobility and appearance of the anterior chamber structures should follow, including the assessment for aqueous flare. The attachment of the iridocorneal angle pectinate ligaments to Descemet’s membrane (i.e., gray line) can be observed medially and laterally in the adult horse (Fig. 1-6) and allows for direct visualization of the horse’s iridocorneal angle. Collection of cultures and Schirmer tear values are performed when indicated prior to placing any medications into the eyes. When indicated, cytology is collected next, usually after application of 0.5% proparacaine hydrochloride (HCl) topical anesthetic (Alcaine 0.5% [Alcon Laboratories, Fort Worth, TX]). Fluorescein staining of the cornea is then performed. Examination of the nasolacrimal system, third eyelid, and conjunctiva is performed concurrently. Fluorescein staining is followed by induction of topical anesthesia with proparacaine if not already given to collect cytology and perform tonometry. The ocular media (cornea, aqueous humor, lens, and vitreous) are evaluated for clarity and transparency by transillumination and ophthalmoscopy.4,9 The anterior surface of the third eyelid can be examined by gently retropulsing the globe to produce passive prolapse of the nictitans. For evaluation of the posterior surface, the third eyelid can be gently grasped with Graefe fixation forceps or manipulated with a strabismus hook.
For complete examination of the lens and posterior segment, mydriasis is required. The most common mydriatic used is tropicamide (Mydriacyl 1% [Alcon]), which takes effect in approximately 10 to 20 minutes and lasts 4 to 6 hours.12,13 In the case of severe intraocular inflammation or reflex uveitis because of corneal disease or trauma, a single application of tropicamide may not be sufficient to dilate the pupil. Topical phenylephrine (2.5% or 10%) does not cause mydriasis in normal horses, nor does it enhance the mydriatic effect of tropicamide.14,15 The use of atropine for routine examination is not recommended because of its longer duration of action and potential adverse effects in the horse.11,16 After mydriasis has been achieved, the clarity, position, and size of the lens, vitreous body, optic nerve, retinal blood vessels, and the tapetal and nontapetal fundus are evaluated. With full mydriasis, the edge of the lens and attachment of the zonular fibers may be visible.17
Vision Testing
Vision testing in horses is subjective. Environmental observation, menace response, dazzle reflex, and maze testing provide only rough data. Determining total blindness is possible with these tests, but determining whether a horse has decreased vision is not easily done. Advanced diagnostic testing such as electroretinography (see later), may help determine if there are abnormalities in retinal electrical function but do not test vision per se. If visual function is in doubt in one or both eyes, the horse can have a unilateral blindfold and be subjected to a maze test. However, horses that are depressed, ataxic, or have vestibular disease may stumble over objects despite having vision.9 Equine vision and vision testing are discussed more extensively in Chapter 11.
Menace Response
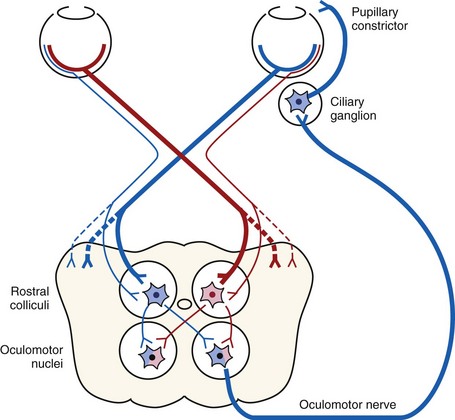
The menace response is a learned protective response in which a menacing movement toward the eye results in closure of the eyelids and possibly retraction of the globe or an avoidance movement of the head.12 The threatening movement can be performed with the examiner’s hand, but care should be taken to avoid contacting the vibrissae and to avoid causing an air current that could be detected even in a blind eye. For detection of a visual deficit in one field, the menacing gesture is directed first toward the nasal visual fields and then toward the temporal visual fields.18 However, partial visual deficits can be extremely difficult to detect using a menace response. The afferent arm of the menace response is the retina and CN II, and the efferent arm is the palpebral branch of CN VII, which innervates the orbicularis oculi muscle (Fig. 1-7).19 A horse that has intact vision but is extremely stoic, depressed, or frightened may have a diminished menace response. Lightly tapping the medial or lateral canthus before attempting to induce the menace response again may heighten the response from an uninterested, stoic horse. A pathologic lack of menace response may result from a lesion in the retina, CN II, the visual cortex, or CN VII (see Fig. 1-7).19 Cerebellar disease can also cause bilateral deficiency in the menace response in the absence of blindness or CN VII paralysis, possibly because of a loss of cerebellar modulation of cerebral visual function.18,19
Pupillary Light Reflexes
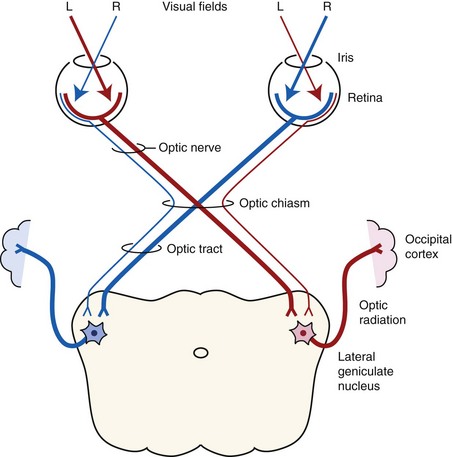
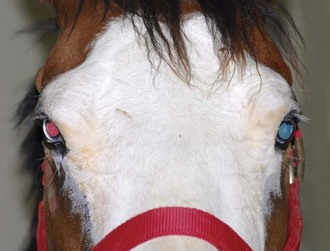
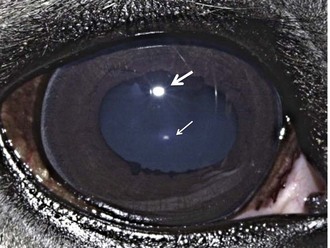
Both eyes should be examined for pupil size and symmetry and for abnormalities such as synechia that may affect the PLR. One method to observe pupil symmetry in the horse is to use an indirect or direct ophthalmoscope directed at the center of the horse’s head from a distance of 6 to 8 feet. This technique will illuminate the pupils via both tapetal reflexes and make it possible to evaluate pupil symmetry (Figs. 1-8 and 1-9).20 Anisocoria may be a normal finding in horses with bilateral heterochromia iridis or unilateral heterochromia iridis in which the larger pupil is ipsilateral to the heterochromic eye (Fig. 1-10).6
When stimulated by light during the PLR, vertical movement of the pupil is much faster and excursion is greater than horizontal movement. The pupil’s shape is a horizontal ellipse that becomes rounder when dilated (Figs. 1-11 and 1-12).21 Pupillary light reflexes can be used to simultaneously evaluate function of the retina, CN II, midbrain, and CN III.18–20 Light directed into one eye should result in constriction of both that pupil (direct response) and the pupil of the contralateral eye (indirect or consensual response). This results from bilateral excitation of the parasympathetic component of CN III in the pretectal region (see Fig. 1-7).18–20 The normal equine pupil responds somewhat sluggishly and incompletely to light, in a biphasic manner.8,19 The first part is a brisk but small reaction, followed by the second slower complete movement. The magnitude and time of response depends on the brightness of the light source and the mental state of the horse. A very focal and bright light source is required to stimulate a rapid and complete response. Pupillary light responses are most vigorous if the beam is directed towards the visual streak in a direction that is temporal (lateral) and slightly dorsal to the optic disc. Consensual responses can be difficult to evaluate in the horse because they tend to be weaker than the direct response and can be awkward for an examiner to determine alone. The indirect PLR is less prominent because of decussation at the chiasm (75%) in the horse, which results in more efferent pupillomotor fibers that return to the ipsilateral side of the brain (see Fig. 1-7).6,20 This is referred to as dynamic contraction anisocoria.17 Evaluation for the consensual light reflex is unnecessary if the horse has vision and a direct response in both eyes. The consensual light reflex can be extremely valuable in evaluating problems when the posterior segment cannot be visualized (e.g., corneal edema, hyphema) for assessment of retinal function in the affected eye. Pupillary escape, a slight dilation that follows constriction under direct light stimulation, is a normal response in the horse.20
Dazzle Reflex
The dazzle reflex, in contrast to the cortically mediated menace response, is a subcortical reflex that requires function of the retina, CN II, CN VII, rostral colliculus, possibly the supraoptic nuclei of the hypothalamus, and orbicularis oculi.18–20 A very bright focal light source is directed into the eye, and blinking or blepharospasm is a normal response. Care should be taken if the light source generates heat, because this can be detected by the horse if the light source is close to the cornea and can also result in blepharospasm.
Palpebral Reflex
Horses normally blink approximately 5 to 25 times per minute at rest.22 The blink is synchronous between both eyes approximately 30% to 100% of the time.22,23 Two types of normal blinking occur in the horse at rest: complete and incomplete.23 Incomplete blinking is most common and consists predominantly of upper eyelid motion downward.23 Complete blinking is associated with an upward movement of the lower lid to meet the upper lid and is highly variable in occurrence.23 The blink rate slows when the horse is sedated, anxious, or focused on an object of interest.
The palpebral reflex is elicited by touching the medial and lateral canthi and results in closure of the eyelids.18–20 If CN V or CN VII is abnormal (e.g., facial nerve paralysis) or if the eyelids are unable to close (e.g., with severe trauma and swelling), the blink may be absent or incomplete.
Corneal Reflex
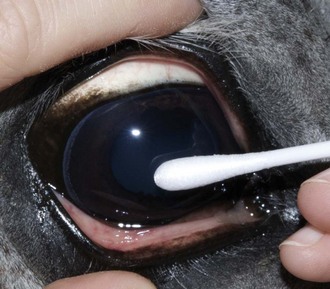
The corneal reflex is elicited by lightly touching the unanesthetized cornea with a sterile cotton-tipped swab and results in closure of the eyelid and retraction of the globe.18–20 This subcortical reflex occurs in response to a tactile or painful stimulus to the cornea. The afferent pathway of the corneal reflex is via the ophthalmic branch of CN V.20 The result should be closure of the eyelid and retraction of the globe, mediated by CN VII and CN VI, respectively.20 If CN V or CN VII is abnormal or if the eyelids are unable to close, the blink may be absent or incomplete. Corneal sensitivity can be quantitated by a technique called corneal esthesiometry, which is described later in this chapter. Lack of corneal sensation may be the cause of corneal ulceration and other corneal abnormalities (see Chapter 5).
Autonomic (Sympathetic and Parasympathetic) Nervous System
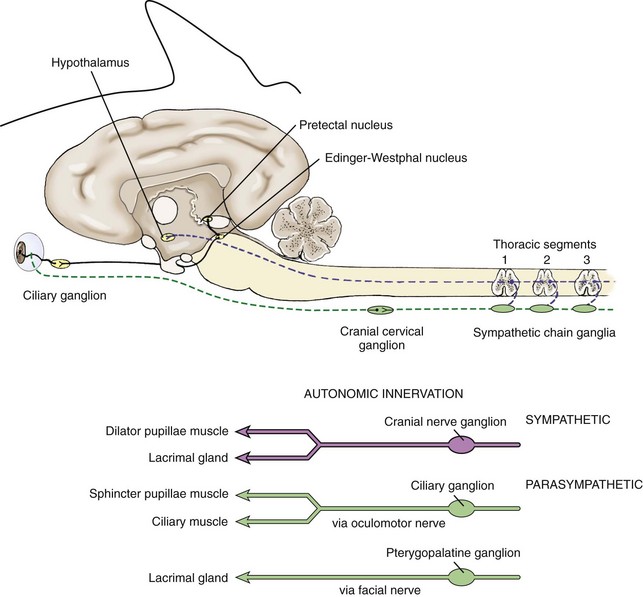
The autonomic nervous system is composed of two main divisions: the sympathetic and parasympathetic nervous systems. The sympathetic nervous system (Fig. 1-13) begins in the hypothalamus and extends down the intermediolateral cell columns of the spinal cord to synapse at C8 to T3. The axons from these cells exit the anterior nerve root of the spinal cord through the white ramus. The fibers (preganglionic fibers) course through the brachial plexus, through the thoracic inlet, ascend in the sympathetic trunk (with the jugular vein), and synapse at the cranial cervical ganglion. Postganglionic fibers proceed rostrally between the tympanic bulla and petrous temporal bone through the orbital fissure and form the sympathetic root of the ciliary ganglion.18–20 Short ciliary nerves (along with contributory fibers to the long ciliary nerves) extend from the ciliary ganglion to the dilator muscle of the iris (see Fig. 1-13).
The sympathetic nervous system controls dilation of the pupils and other motor functions of the eyes and face. Damage to sympathetic innervation to the head may result in Horner’s syndrome (miosis, ptosis, enophthalmos, increased sweating on the face and ear of the affected side, ipsilateral distention of facial blood vessels, and ipsilateral hyperemia of the conjunctiva and nasal mucosa).24–30 Chapter 13 offers a detailed description of the clinical signs and diagnosis of Horner’s syndrome in horses. Causes reported in the horse include jugular vein and carotid artery injections, cervicothoracic spinal cord injury, cervical abscesses, guttural pouch disease or surgery, neoplasia or trauma of the neck and thorax, trauma to the vagosympathetic trunk, middle ear disease, traumatic lesions of the basisphenoid area, polyneuritis equi syndrome, equine protozoal myelitis of the cervical spinal cord, esophageal rupture, and trauma to the neck and thorax.*
The parasympathetic nervous system to the eye originates in the oculomotor nucleus in an area called the Edinger-Westphal nucleus. The fibers from this nucleus travel with the oculomotor nerve (CN III) and exit in the motor root of the ciliary ganglia where they synapse. The postsynaptic fibers travel to the eye in the short ciliary nerves to the constrictor muscles of the iris (see Fig. 1-13). Parasympathetic fibers also run with the facial nerve (CN VII) to the lacrimal gland to result in lacrimation when stimulated.
Restraint and Sedation
Some horses can undergo an ocular examination without sedation, but most horses require sedation for a complete, detailed ophthalmic examination. Use of restraint and sedation depends on the temperament of the horse, availability of equipment, and comfort level of the handlers and examiner. Tranquilization with detomidine HCl (Dormosedan [Pfizer Animal Health, New York, NY]) 0.02 to 0.04 mg/kg, administered intravenously [IV]) is preferred for ophthalmic examinations because it provides rapid tranquilization without an excitation phase (either on induction or during recovery) and a steady and low head position without movement (i.e., fine tremors). Xylazine 0.5 to 1 mg/kg IV with or without butorphanol tartrate (Torbugesic [Fort Dodge Animal Health, Overland Park, KS] 0.01 to 0.02 mg/kg IV) can also be used.8,9 Butorphanol is commonly added for painful procedures or additional restraint.8,9 However, xylazine can have a profound excitation phase on induction or recovery. Both butorphanol and xylazine are associated with fine head tremors, or head “jerks,” which can be very disruptive during slit-lamp biomicroscopy and minor procedures around the eye such as cytology collection. Addition of acepromazine (0.02 to 0.04 mg/kg IV; 20 mg IV in a 500-kg horse)32 10 to 15 minutes prior to the detomidine is recommended to avoid the fine tremors when additional tranquilization is needed for extended examination or minor standing surgical procedures. Additional restraint includes lip (rope or chain) and neck twitch (manual). Although these techniques are generally very effective for short-term restraint (i.e., less than 10 minutes), some horses react adversely to these techniques, so caution is advised when using them.
Regional Nerve Blocks
Two ophthalmic nerves are frequently denervated (“blocked”) during the equine ocular examination: the auriculopalpebral, or more precisely the palpebral branch of the facial nerve (CN VII), and the frontal (supraorbital) branch of the trigeminal nerve (CN V).33,34 When these nerves are blocked, akinesia and anesthesia, respectively, of the upper eyelid occurs.
Regional Akinesia
The most common nerve blocked is the palpebral branch of the auriculopalpebral nerve, which innervates the orbicularis oculi muscle, responsible in part for eyelid closure. The orbicularis oculi muscle in horses is very strong, and therefore akinesia of this muscle is required to open the eyelid for examination in many horses, especially horses that are painful. It is extremely important in conditions in which the structural integrity of the globe is compromised, because the pressure applied by the muscle during manipulation for examination or during blepharospasm could result in rupture of the globe. Akinesia of the eyelids may be induced for routine eye examination, diagnostic procedures (e.g., corneal cytology and culture), therapy (e.g., subconjunctival injections, placement of a subpalpebral lavage), and standing surgeries.33,34
A volume of 1 to 2 mL of an anesthetic is injected subcutaneously with a 25-gauge,  -inch needle adjacent to the nerve, and the injection site is massaged to facilitate anesthetic diffusion.8,9,12,33,34 Anesthetics most frequently used for eyelid blocks include 2% lidocaine HCl, which has an onset of action of 4 to 6 minutes and a duration of 60 to 90 minutes, and 2% mepivacaine (Carbocaine [Pharmacia & Upjohn Company, Division of Pfizer Inc., New York, NY]), with an onset of action of 3 to 5 minutes and a duration of 90 to 120 minutes.8,9,12 Procaine or bupivacaine can also be used. Repeated injections of anesthetic may result in a refractory phenomenon, requiring higher volumes of drug and longer times to achieve akinesia.12 The auriculopalpebral nerve block results in paralysis of the orbicularis oculi muscle of the upper eyelid and variable paralysis of the lower eyelid for approximately 1 to 2 hours.8,9,12 Duration of anesthesia can be prolonged with the addition of 1:10,000 epinephrine,12 but this is not usually required for most examinations or minor surgical procedures. Ptosis, narrowing of the palpebral fissure, and easy manual elevation of the upper eyelid should result.8,9,12 Sensation to the eyelids and some palpebral function remains intact, so the horse can usually blink and continue to protect the cornea. Once the examination is concluded, if the horse is not blinking well, topical ophthalmic ointments should be used every 30 minutes to protect the cornea until palpebral function returns.
-inch needle adjacent to the nerve, and the injection site is massaged to facilitate anesthetic diffusion.8,9,12,33,34 Anesthetics most frequently used for eyelid blocks include 2% lidocaine HCl, which has an onset of action of 4 to 6 minutes and a duration of 60 to 90 minutes, and 2% mepivacaine (Carbocaine [Pharmacia & Upjohn Company, Division of Pfizer Inc., New York, NY]), with an onset of action of 3 to 5 minutes and a duration of 90 to 120 minutes.8,9,12 Procaine or bupivacaine can also be used. Repeated injections of anesthetic may result in a refractory phenomenon, requiring higher volumes of drug and longer times to achieve akinesia.12 The auriculopalpebral nerve block results in paralysis of the orbicularis oculi muscle of the upper eyelid and variable paralysis of the lower eyelid for approximately 1 to 2 hours.8,9,12 Duration of anesthesia can be prolonged with the addition of 1:10,000 epinephrine,12 but this is not usually required for most examinations or minor surgical procedures. Ptosis, narrowing of the palpebral fissure, and easy manual elevation of the upper eyelid should result.8,9,12 Sensation to the eyelids and some palpebral function remains intact, so the horse can usually blink and continue to protect the cornea. Once the examination is concluded, if the horse is not blinking well, topical ophthalmic ointments should be used every 30 minutes to protect the cornea until palpebral function returns.
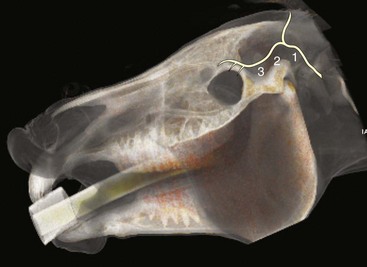
The auriculopalpebral nerve branches from the main trunk of the facial nerve, where it is protected by the parotid gland for the full length of the caudal border of the ramus of the mandible.12,20 It then emerges from beneath the gland just caudal to the caudal border of the condyle of the mandible, where it is covered by thin facial muscles and lies close to the rostral auricular artery and vein.12 The branches then pass rostrally and dorsally to reach their destination (Fig. 1-14).
The auriculopalpebral nerve can be blocked subfascially in the depression just anterior to the base of the ear where the caudal border of the coronoid process of the mandible meets the zygomatic process of the temporal bone. At this point, the nerve emerges from the parotid salivary gland and becomes subcutaneous on the lateral aspect of the dorsal tip of the coronoid process (see Fig. 1-14).12,33,34
The palpebral branch of the auriculopalpebral nerve can be blocked just lateral to the highest point of the caudal zygomatic arch, where the nerve can be palpated through the skin by running a finger forcefully over the dorsal border of the bone (Fig. 1-15, see Fig. 1-14).33,34 The palpebral branch of the auriculopalpebral nerve can also be blocked where it lies on the zygomatic arch caudal to the bony process of the frontal bone (see Fig. 1-15).33,34
Regional Anesthesia and Analgesia
Sensation to the eyelids is provided by the ophthalmic and maxillary divisions of the trigeminal nerve (CN V) (Fig. 1-16).12 The frontal, lacrimal, and infratrochlear nerves arise from the ophthalmic branch of CN V, and the zygomatic nerve arises from the maxillary branch of CN V.6,12 The frontal (supraorbital) nerve innervates most of the central upper eyelid and is the only sensory block normally required for examination.12 The lacrimal nerve provides sensory innervation for the lateral upper eyelid.12 The infratrochlear nerve provides sensory innervation for the medial canthus.12 The zygomatic nerve innervates the majority of the lateral lower eyelid.12 The nasociliary nerve, a branch of the maxillary branch of CN V, provides sensory innervation to the cornea.12 Anesthesia of these nerves is sometimes necessary for eyelid and conjunctival biopsies or simple surgeries, as well as subpalpebral lavage placement in the horse. The anesthetics most frequently used are the same as those used for akinesia and include lidocaine HCl and mepivacaine.
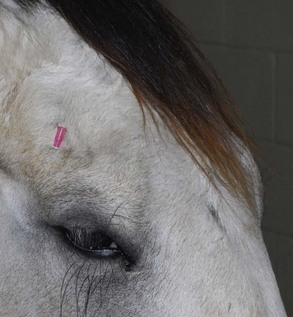
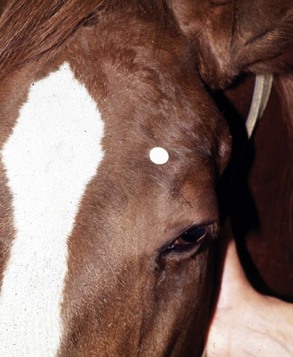
The four main sensory nerve branches can be blocked directly as follows: The frontal (supraorbital) nerve is blocked as it emerges from the supraorbital foramen within the frontal bone (Figs. 1-17 and 1-18).12,17 This foramen can be palpated if the examiner places his or her thumb below the dorsal orbital rim and the middle finger in the supraorbital fossa. The examiner then places the index finger straight down midway between the thumb and middle finger to locate the supraorbital foramen (see Fig. 1-18). A depression is usually palpable. A 25-gauge, 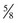 -inch needle is then inserted subcutaneously over the foramen, and 1 to 2 mL of anesthetic is injected (Fig. 1-19). Passing the needle into the foramen is not recommended because this may damage the supraorbital artery and vein, which exit the skull through the supraorbital foramen. Furthermore, if the needle inadvertently enters the periosteum surrounding the supraorbital foramen, this can be painful and the horse may react negatively. The frontal nerve is mainly sensory, but this block can result in partial upper eyelid akinesia as well, likely by further denervating the branches of the palpebral nerve.9
-inch needle is then inserted subcutaneously over the foramen, and 1 to 2 mL of anesthetic is injected (Fig. 1-19). Passing the needle into the foramen is not recommended because this may damage the supraorbital artery and vein, which exit the skull through the supraorbital foramen. Furthermore, if the needle inadvertently enters the periosteum surrounding the supraorbital foramen, this can be painful and the horse may react negatively. The frontal nerve is mainly sensory, but this block can result in partial upper eyelid akinesia as well, likely by further denervating the branches of the palpebral nerve.9
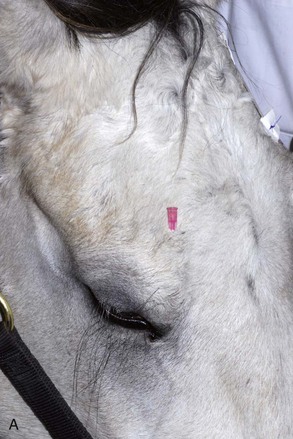
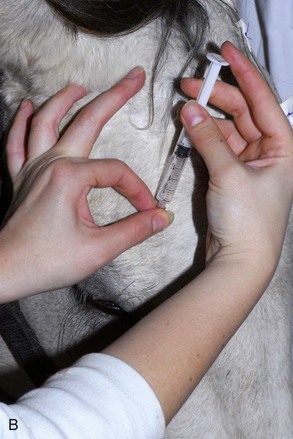
Figure 1-19 A, A 25-gauge, 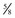 -inch needle is inserted into or just over the foramen. B, Inject 1 to 2 mL of anesthetic.
-inch needle is inserted into or just over the foramen. B, Inject 1 to 2 mL of anesthetic.
The lacrimal nerve can be blocked by injecting 1 mL of lidocaine adjacent to the lacrimal notch, a depression that can be palpated on the dorsolateral boney orbital rim, or by using a line block along the lateral third of the dorsal orbital rim (Fig. 1-20 and see Fig. 1-16).
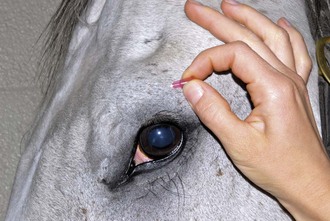
Figure 1-20 The lacrimal nerve can be blocked by using a line block along the lateral third of the dorsal orbital rim.
The zygomatic nerve can be blocked with a line block along the ventrolateral orbital rim (Fig. 1-21 and see Fig. 1-16).
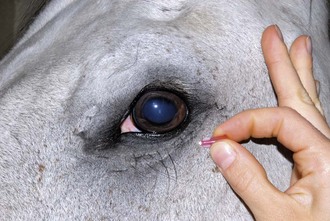
Figure 1-21 The zygomatic nerve can be blocked with a line block along the ventrolateral orbital rim.
The infratrochlear nerve can be blocked as it runs through the trochlear notch located medially on the dorsal orbital rim (Fig. 1-22 and see Fig. 1-16). The notch can be palpated along the orbital rim.
Globe and Orbit Examination
Anatomy of the globe and orbit is reviewed in detail in Chapter 3. Initial examination of the globe and orbit should be made with the examiner positioned in front of the horse (see Fig. 1-4), where symmetry between the eyes is carefully assessed. Palpebral fissure size and symmetry, relative globe position, and direction of the eyelashes are evaluated. The upper eyelashes of the healthy horse are nearly perpendicular to the cornea (see Fig. 1-5).8 A change in the angle between the eyelashes and the cornea may indicate blepharospasm, enophthalmos, exophthalmos, or ptosis (see Fig. 1-5).8
Apparent changes in globe size (e.g., hydroophthalmus [Fig. 1-23]) should be differentiated from changes in globe position (e.g., exophthalmos [Fig. 1-24]). Cornea globosa (Fig. 1-25) has been reported in the Rocky Mountain horse and may be difficult to distinguish from hydroophthalmus.35,36 The orbit should be examined by observation, palpation of the bony orbital rim, and retropulsion of the globe through a closed eyelid.4,8,9 Forceful manipulation of the eyelid and retropulsion should not be performed if the structural integrity of the cornea or globe may be compromised.
Eyelids and Conjunctiva Examination
Anatomy of the eyelids and conjunctiva is reviewed in detail in Chapter 4. Examination of the eyelid should include assessment of function and detailed examination using diffuse illumination with magnification (e.g., using a slit-lamp biomicroscope). The periocular tissues including the eyelids, conjunctiva, sclera, and nictitans should be inspected with transillumination,17 the technique of direct focal illumination for inspection of the anterior structures of the eye. It can be performed with a Finnoff transilluminator (Welch-Allyn, Skaneateles Falls, NY), direct ophthalmoscope, slit-lamp biomicroscope, or even a penlight. Eyelids should be examined for position, movement, and conformation prior to the use of eyelid blocks.4,8,9 Attempts to forcefully elevate the upper eyelid should be avoided if a palpebral nerve block has not yet been performed, and each eye should be examined with minimal handling of the adnexal tissues. Culture and cytology should be collected prior to instillation of any medications (see detailed information on culture and cytology in this chapter and in Chapter 2). Biopsy of the eyelid or conjunctiva should be considered if indicated (see later and in Chapter 4).
Lacrimal and Nasolacrimal System Examination
Please see anatomy of the lacrimal and nasolacrimal system in Chapter 4. Assessment of the lacrimal system is done by microscopic examination of the tear film via slit-lamp biomicroscopy (or other means of magnification) and adjunctive diagnostic tests, such as the Schirmer tear test. The examiner should also inspect the openings of the proximal (eyelid) and distal (nasal) nasolacrimal puncta (Fig. 1-26).
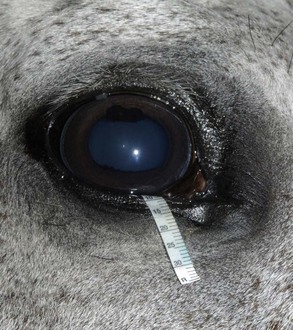
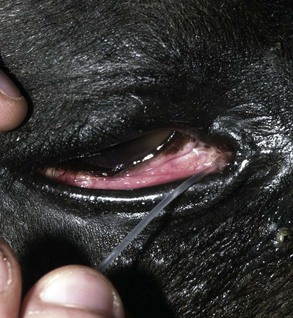
The Schirmer tear test (STT) is used commonly to measure aqueous tear production.37–40 In the test, a filter paper strip is placed in the conjunctival sac, and wetting is then measured in millimeters per 60 seconds (Fig. 1-27). Commercial filter paper strips available include standardized Sno-Strips (Akorn) and Color Bar (Eagle Vision/Schering-Plough, Kenilworth, NJ). Strips can also be made from Whatman filter paper (No. 40, 5 × 40 mm with a notch 5 mm from the end).12 The Schirmer I test, in which no topical anesthesia is used, measures the approximate amount of basal and reflex tearing. The Schirmer II test, performed after the application of topical anesthesia, theoretically only measures basal secretion of aqueous tears. Some residual tear volume may make both of these measurements slightly inaccurate. The STT should be performed before manipulation of the eye and orbit during examination to minimize reflex tearing. There are no reports of the effect of an auriculopalpebral nerve block on the STT in horses.
Deficiencies in aqueous tear production have rarely been reported in the horse.23,38,40–46 Possibly this is because the STT is not a part of the routine ophthalmic examination in the horse, but it should be. Specific indications to perform STT include evidence of CN VII dysfunction (e.g., after trauma, facial paralysis), desiccated cornea or conjunctiva, presence of tenacious mucoid discharge, and presence of unexplained corneal vascularization or ulceration. Keratoconjunctivitis sicca is most commonly the result of CN V or VII trauma but has also been reported in cases of fractures of the mandible and stylohyoid bone, post anesthesia, locoweed poisoning, eosinophilic dacryoadenitis, hypothyroidism, and in association with corneal stromal sequestration.23,38,40–46
The effects of age, season, gender, environment, sex, time of day, and placement of strips on STT results in healthy horses and ponies have also been reported.37,39 In general, the STT value in the horse is much greater than that in cats and dogs.38,47,48 STT values are highly variable between eyes and between the same eye during different times of the day, and this appears to be unrelated to signalment, housing, or season.37 One study found a diurnal variation in horses housed in a 12-hour light and 12-hour dark setting.39 These horses had STT values that gradually increased during the light phase, peaked at 4 to 6 hours, then decreased during the dark phase.39 Healthy horses have been reported to have an STT I range of 11 to greater than 30 mm wetting/min and 15 to 20 mm/30 sec. Both sick and healthy neonatal foals have been reported to have lower STT values than adults.49,50 STT I values for sick neonatal foals (14.2 ± 1 mm of wetting/min) and healthy neonatal foals (12.8 ± 2.4 mm/min) were not significantly different but were lower than STT I values from healthy adult horses (18.3 ± 2.1 mm/min).49,50
Comparisons of STT I and STT II values revealed minimal differences in one study (i.e., STT I and STT II values of 12.7 ± 9.1 mm wetting/min and 9.9 mm ± 4.25 mm wetting/min),48 while the second did not reveal a difference between STT I and STT II values.37 This is in contrast to the dog, in which the STT I value is significantly higher than the STT II value.51 Sedation with xylazine does not affect the STT value; however, general inhalant anesthesia with halothane does lower the STT value for up to 3 hours.38
Borderline STT measurements (e.g., measurements of 10 to 15 mm wetting/min) should always be repeated. Comparison of tear test results between the two eyes should be cautiously interpreted in clinical assessment of decreased tearing.37 In general, repeatable measurements of less than 10 mm wetting/min should be considered abnormal in conjunction with clinical signs.4 Please see Chapters 4 (adnexa and lacrimal) and 5 (cornea) for more information on keratoconjunctivitis sicca.
Nasolacrimal Duct Patency
The physiologic patency of the nasolacrimal system can be evaluated with topical sodium fluorescein, which is not rinsed from the eye.12 Passage of the fluorescein to the distal puncta in the nares (Jones test) is timed and should occur within 5 minutes but may take up to 20 minutes (Fig. 1-28).17 The required time for passage is influenced by the amount of fluorescein placed, tear production, and length of the individual horse’s nasolacrimal system.17 A positive test result is definitive for a patent nasolacrimal duct but does not prove that both proximal puncta are patent.17 A negative test result is only suggestive of a problem and may even be normal in the horse because of the large volume capacity of the nasolacrimal duct.17,52,53 However, the nasolacrimal duct should be irrigated if the dye fails to appear and clinical signs suggest a problem such as epiphora (watery ocular discharge) without an obvious cause, mucopurulent ocular or nasal punctal discharge, or dacryohemorrhea.17,52,53
Irrigation of the nasolacrimal duct can be performed retrograde (i.e., from the distal nares opening) or normograde (i.e., from the proximal eyelid puncta).12 Sedation is usually required to perform either procedure in the horse. Retrograde irrigation through the distal opening to the nasolacrimal duct is easiest to perform (Fig. 1-29) because of the larger size of the opening.17,53 The distal nasolacrimal puncta can usually be cannulated by a No. 5 or 6 polyethylene urinary catheter.17 Suitable catheters are 4 to 6 Fr canine urinary catheters, 5 Fr feeding tubes, or polyethylene tubing.17 The largest catheter that will pass through the bony canal in an adult is a 6 Fr urinary catheter.17 The tip of the catheter, after it has been coated with lidocaine gel, is inserted into the distal punctal opening for a distance of at least 5 cm. Digital pressure should be applied to the opening to close it and prevent normograde loss of fluid. A 12- to 20-mL syringe previously filled with eyewash is attached, and gentle irrigation of the nasolacrimal duct is performed until fluid exits the proximal punctum near the medial canthus of the eye. Sneezing by the horse is common during this procedure and may be violent. A list of supplies needed to perform nasolacrimal duct irrigation can be found in Box 1-3.
Box 1-3 | Supplies Needed for Irrigation of the Nasolacrimal Duct
Retrograde Irrigation
If retrograde irrigation is unsuccessful, then normograde irrigation from the proximal puncta should be attempted with a lacrimal canula, open-ended tomcat catheter, or teat tube syringe (Fig. 1-30).17 The puncta in the lower eyelid are usually slightly larger and easier to cannulate than the puncta in the upper eyelid.17 Gentle pulse pressure may be required to unblock an obstructed duct. Excessive force in the placement of the catheter or during irrigation should be avoided because significant damage to the nasolacrimal duct could result.17 Ducts that are compromised by a foreign body or other anatomic obstruction (e.g., after trauma, mass effect) may not be effectively irrigated.54 Skull radiographs or CT scans and a contrast dye study (e.g., dacryocystorhinography) should be performed next if the duct cannot be irrigated. See descriptions of these diagnostic techniques later in the chapter.
Cornea and Sclera Examination
The anatomy and diseases of the equine cornea are described in Chapter 5. To examine the cornea of the horse, diffuse and focal direct illumination (or transillumination) with magnification is used first, followed in most cases by biomicroscopy using a slit-lamp. The corneal examination should be performed with the observer located rostral to the eye. Light directed diagonally across the cornea will reveal opacities of the cornea against the dark background of the pupil.17
The Purkinje-Sanson reflexes are three reflections from the eye produced by the light source during transillumination (Fig. 1-31).17 Disease may alter the sharpness and location of these reflexes. The first, largest, and most anterior originates from the cornea. The second originates from the anterior lens capsule, and the third and most posterior originates from the posterior lens capsule. If a slit-lamp biomicroscope is used, two corneal reflexes are seen, one from the anterior surface and the other from the endothelium.17 The corneal and anterior lens capsule reflexes are virtual and noninverted and will move in the same direction as a change in the light position. The image on the posterior surface is real and inverted and will move in the opposite direction to the light.17 The images are valuable in determining corneal clarity, depth of the anterior chamber, thickness and position of the lens (after mydriasis), and in locating lesions within the lens.17
Biomicroscopy for Corneal Exam
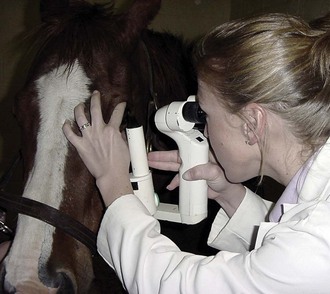
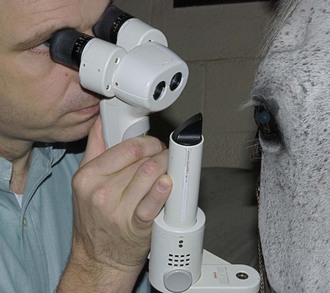
The technique of biomicroscopy, in which a slit-lamp binocular microscope with an external pivoting light source is used, is the same for horses as for humans and small animals and has been well described elsewhere.12,55–58 Slit-lamp biomicroscopy improves visualization and localization of lesions of the cornea, anterior chamber, lens, and anterior vitreous by means of transillumination and retroillumination.12 It can also be used to assess corneal thickness (i.e., pachymetry), anterior chamber depth, and aqueous flare.12
The availability of portable handheld models of the slit-lamp biomicroscope has made biomicroscopy for equine ophthalmology easy and efficient. Portable models are available from Clement-Clark, Kowa, Nippon, Dioptrix, and Zeiss. The Kowa SL-14 or SL-15 (×10 or ×16 magnification) is light and powered by a rechargeable battery, and therefore of excellent use in examination of a horse (Fig. 1-32). However, lack of magnification above ×16 and inherent movement of the examiner and horse limits the ability to see fine structure and lesions. An alternative to a biomicroscope is using magnification (i.e, ×2.3 magnifying head loupes) and the slit beam on the direct ophthalmoscope. Very small “slit-lamps” are also made by Heine (HSL 150 [Heine USA, Dover, NH]; Eidolon Hand Held Slit Lamp Model 510L [Eidolon Optical LLC, Natick, MA]) that resemble a penlight with a magnifier on the end (Fig. 1-33). Although these instruments are inexpensive and portable, their lack of magnification and illumination limits their usefulness.12
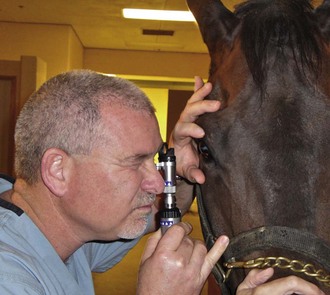
Figure 1-33 Use of a small, portable slit-lamp (Heine Handheld Slit Lamp, Heine USA, Dover, NH).
(Photograph courtesy Dr. David Wilkie.)
The light beam of the biomicroscope should be angled at 20 to 45 degrees from the axis of the microscope and thus the visual plane of the observer (Fig. 1-34). The light beam width, length, orientation, and color can then be modified by a series of diaphragms and filters.12 The focal distance of the instrument is 7 to 10 cm, and fine focus is achieved by moving either toward or away from the eye within this range.12
The initial examination of the horse should proceed with diffuse illumination: a wide, low-intensity slit beam should be used, and the microscope should be defocused from the light.12 The surfaces of the eyelids, cornea, conjunctiva, and iris should be inspected. With the use of low magnification, a broad slit beam is focused on the cornea, creating a parallelepiped (i.e., a three-dimensional section) of illuminated tissue.12,55 This allows visualization of transparent structures such as the cornea and lens in three dimensions. In the cornea, the anterior surface, stroma, and posterior surface of the cornea can be visualized.55 Nontransparent structures such as the sclera only yield a magnified two-dimensional surface or external view. The slit beam is then narrowed and intensified to reveal a two-dimensional cross-section of the cornea and lens, allowing the examiner to accurately determine lesion depth and axial positioning.55 This is extremely important in evaluating the depth of corneal lesions (e.g., stromal ulcerative keratitis, stromal abscesses) in the horse.
Direct and indirect retroillumination are performed by reflecting the slit beam from deeper structures while focusing on more superficial structures.55 Other techniques that can be performed with slit-lamp biomicroscopy, such as specular reflection, are difficult to impossible in a horse because of continuous slight ocular movements.
Culture of Corneal Lesions
Culture and sensitivity testing can aid in the diagnosis of infectious keratitis and help to determine antimicrobial therapy. Specimens for culture should be obtained as early as possible in the examination, before the administration of topical preparations (e.g., proparacaine, fluorescein). Because topical anesthesia is routinely used in the workup of painful ocular disease in the horse, it is important to note that some topical drugs (e.g., proparacaine, tetracaine) have been reported to inhibit organism growth.59 However, it has also been shown that a single application of proparacaine is unlikely to affect culture results.60 In reality, some horses are in so much pain that even with sedation and regional nerve blocks, they will not allow the examiner to obtain an appropriate sample without application of topical anesthesia.
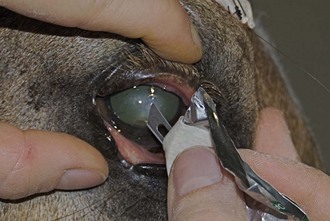
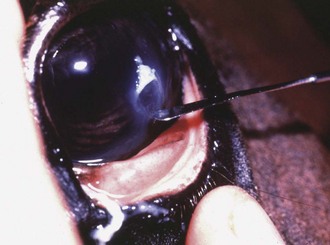
Culture of the ocular surface can be performed with sterile, moistened, Dacron-tipped swabs or the blunt end of a sterile surgical blade. The Dacron-tipped swab is rubbed or rolled (Fig. 1-35) over the area to be cultured, and the blunt end of the surgical blade is used in a scraping motion (Fig. 1-36). The eyelids should be retracted to prevent contamination of the sample if the eyelids are not the object of sample collection. Samples should be taken from the edges of a corneal ulcer to avoid possible deep or fragile areas of the cornea.
Type of culture and choice of antibiotic sensitivities should be made on the basis of clinical signs and the region and environment in which the horse is living. Both aerobic and fungal culture and sensitivity testing are usually indicated for most cases. Anaerobic testing may be indicated in some circumstances, but fungal testing is not necessary in nonendemic areas such as the southwestern United States. Fungal pathogens of the equine eye are usually saprophytic fungi that require enriched media such as Sabouraud dextrose agar or blood agar.8 Fungal or bacterial pathogens can be so deep within the cornea that a diagnostic sample cannot be obtained, especially in the case of a stromal abscess. Culture of equine herpesvirus 2 (EHV-2) from the cornea has also been reported.61,62 Viral culture or isolation usually requires that the sample be placed in a sterile saline solution in an Eppendorf tube, but instructions should be obtained directly from the testing laboratory. See Chapter 5 for more information regarding indications for culture and pathogens to consider for equine keratitis.
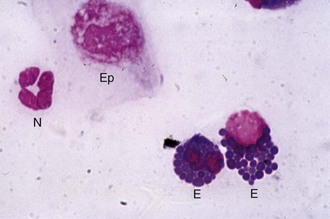
Cytology
Cytology is a quick, simple, and indispensable method for characterizing the type of inflammatory (i.e., neutrophilic, lymphocytic, or eosinophilic) process present and may also assist in making a diagnosis (e.g., bacteria or fungal hyphae). Cytology is indicated in all cases of ulcerative keratitis in the horse. Cytology can provide rapid results that may guide the immediate course of therapy.63–65 Tools for collecting cytologic samples include instruments that are also used to obtain samples for culture (i.e., Dacron-tipped swabs, blunt end of a sterile scalpel blade), as well as cytobrushes and spatulas.8 Topical anesthetic, microscope slides, slide stain, and a microscope are also required. A microscope slide alone can be used to perform “impression” cytology. This method is probably not effective in identifying pathologic organisms in the case of corneal disease but may be helpful in situations such as the presence of an ulcerative eyelid mass.
The cotton-tipped or Dacron-tipped swab provides the least traumatic method of retrieving adequate exfoliative samples.63 This technique is recommended when excessive manipulation is contraindicated (e.g., deep or melting corneal ulceration). Use of a spatula or the blunt end of a scalpel blade is a more precise method of collecting cells from specific areas, and greater numbers of deeper cells can usually be obtained (Fig. 1-37).12 As in the collection of samples for culture, topical anesthetic is usually required regardless of instrument used. The cytobrush has proved to be superior in all cytologic parameters studied when compared with cotton-wool tips and two different spatulas in dogs, cats, sheep, goats, cattle, and horses.63,66 The large size of the cytobrush is a disadvantage in the eyes of small animals but not in the large equine eye. A cytobrush was used to obtain a cytologic sample in a report in which a Histoplasma species was identified in a case of equine keratitis.66
During collection or transfer to slides, care must be taken with all samples to prevent cell damage and gently form a monolayer. The examiner should roll the swab gently, should not “spin” the cytobrush, and should not use excessive force with the blade edge, because this leads to greater cell damage.63 Commonly used stains include the Gram stain and various Romanowsky-type stains (e.g., Diff-Quik, Wright-Giemsa stain) (Fig. 1-38). The Romanowsky-type stains can be used for quick “screening” in cases of ulcerative keratitis when information is needed to formulate an initial therapeutic plan. The Romanowsky-type stains are generally satisfactory for detection of bacteria, fungal hyphae, yeast bodies, inflammatory cells, and neoplastic cells. The Gram stain is indicated to provide further information about identified bacteria. Fungal hyphae may be difficult to identify on routine staining and may require more specialized stains for fungal elements, which include periodic acid-Schiff (Fig. 1-39) and Gomori’s methenamine silver stain (Fig. 1-40).67 In addition to routine staining, newer tests are becoming available for infectious diseases in the horse. These include the polymerase chain reaction (PCR) and immunofluorescent antibody test for herpes (i.e., EHV-1, EHV-2, EHV-4) and fungal DNA on corneal or conjunctival cytologic and histopathologic specimens.68 Careful interpretation of these sensitive tests is required by the clinician, as demonstrated in one study in which EHV-2 DNA was detected by PCR in 22 out of 77 (28.6%) ocular swabs of normal horses and positive in only 4 out of 48 (8.3%) samples from diseased horses.69 Topical fluorescein staining can interfere with test results (i.e., cause false-positive results), and therefore samples for immunofluorescent antibody testing should be collected before fluorescein staining.70 For more information on collecting and interpreting ocular cytology, see Chapter 2.

 -inch, 19-gauge spinal needle for retrobulbar block
-inch, 19-gauge spinal needle for retrobulbar block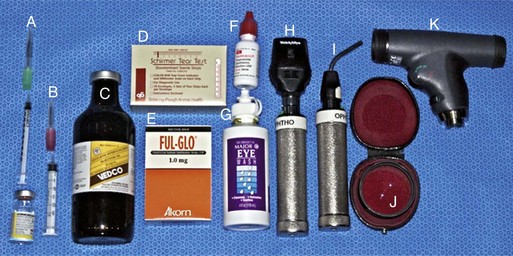
 -inch needle, 25-gauge, 1-inch needle, 1 mL and 3 mL syringes. B, Tranquilizer (detomidine). C, 2% Lidocaine HCl. D, Schirmer tear test strips. E, Fluorescein dye strips. F, 1% Tropicamide HCl. G, Sterile eyewash. H, Direct ophthalmoscope. I, Finnoff transilluminator. J, Indirect ophthalmoscopy lens (20 Diopter). K, Panoptic ophthalmoscope.
-inch needle, 25-gauge, 1-inch needle, 1 mL and 3 mL syringes. B, Tranquilizer (detomidine). C, 2% Lidocaine HCl. D, Schirmer tear test strips. E, Fluorescein dye strips. F, 1% Tropicamide HCl. G, Sterile eyewash. H, Direct ophthalmoscope. I, Finnoff transilluminator. J, Indirect ophthalmoscopy lens (20 Diopter). K, Panoptic ophthalmoscope.