C for cardiovascular
The prognosis for respiratory arrest without cardiac arrest in reptiles is good. With intermittent positive-pressure ventilation (IPPV) and 100% oxygen, reversal of any anaesthesia and administration of doxapram, recovery is likely.
However, cardiac arrest in reptiles carries a worse prognosis than for mammals. This is partly due to the robustness of the heart in most reptiles, meaning that should arrest occur, there is usually significant myocardial hypoxaemic damage. Most reptiles become bradycardic immediately before arrest, and if this is recognised, rapid administration of atropine at 0.02 mg/kg can be effective. Epinephrine may be given intravenously, intraosseously or often more effectively intratracheally.
D for drugs
See Table 24.1 for a list of commonly used ‘emergency’ drugs in reptiles. It should be noted that most sick reptiles are borderline or fully septicaemic. They tend to be attacked by their own gut bacteria, which are generally Gram negative in nature, and often contain the Salmonella and Pseudomonad bacterial family. Therefore, a bacteriocidal antibiotic with good action against Gram-negative bacteria should be used. These include the fluoroquinolones and third-generation cephalosporins. However, other injuries may be sustained such as dog attacks, and so anaerobic bacteria may also be implanted into wounds (Figure 24.2).
Figure 24.2 Dog-attack wounds in terrestrial chelonians are common and may result in serious trauma and infections.

Table 24.1 Commonly used emergency and recovery medications for reptiles.
| Drug | Dosage | Notes |
| Adrenaline | 0.05–0.5 mL depending on size of reptile | Use intratracheally after intubation and IPPV with 100% oxygen |
| Allopurinol | 10–50 mg/kg PO SID | Reduces uric acid production to aid management of gout |
| Calcium gluconate | 100 mg/kg IM/SC/intracoelomically | Hypocalcaemic tetany especially in female egg-bound green iguanas |
| Ceftazidime (Fortum® Pfizer) | 20 mg/kg SC/IM/IV q72h | Broad-spectrum bacteriocidal third-generation cephalosporin; particularly effective against Gram-negative bacteria |
| Diazepam | 0.2–0.5 mg/kg IM/IV | Muscle necrosis if given IM |
| Doxapram | 0.5 mg/kg PO/IV | Intubate and use IPPV |
| Enrofloxacin | 5–10 mg/kg SID | Licensed antibiotic for reptiles. Useful against Gram-negative bacteria but not against anaerobes. Can cause muscle necrosis |
| Furosemide | 1–5 mg/kg | Diuretic but action not known |
| Hydrochlorothiazide | 1 mg/kg | Diuretic |
| Midazolam | 0.2–0.5 mg/kg IM/IV | Less likely to cause muscle necrosis than diazepam |
| Meloxicam | 0.2–0.3 mg/kg IM/PO SID | Beware use if already has renal damage |
| Oxytocin | Chelonians 10 IU/kg IM Lizards 5–20 IU/kg IM Snakes 20–40 IU/kg IM | Uterine muscle stimulant. May be repeated on max of four occasions. Useful to administer calcium gluconate first |
| Silver sulfadiazine cream (Flamazine® Smith & Nephew) | Topical on burns/wounds | Effective cream against Gram-negative bacteria and some fungi |
| Vitamin B1 | 25–35 mg/kg IM/PO/SC | Thiamine deficiency (fish-eating snakes) |
Garter and water snakes, who are fed salt-water fish which has been previously frozen, may suffer from a relative deficiency of vitamin B1 (thiamine), which can lead to a neurological condition (similar to cerebrocortical necrosis in grain-gorged cattle) manifesting as an inability to right itself and continual star gazing. Injections of vitamin B1 at 25–35 mg/kg may be effective if administered quickly, and sedation with midazolam/diazepam or anaesthesia may be necessary to prevent seizuring (Figure 24.3).
Figure 24.3 Hypovitaminosis B1 is common in garter snakes fed previously frozen salt-water fish and may require sedation with midazolam/diazepam or anaesthesia in addition to vitamin B1 injections to control seizuring and star-gazing neurological signs.

Cardiovascular and respiratory diseases are relatively common, and pneumonia or lung oedema may result. Use of diuretics such as furosemide and hydrochlorothiazide may be helpful. Oxygen therapy can be used, but care should be taken as the impetus for breathing in reptiles is a lowered PaO2 rather than an elevated PaCO2 as in mammals, therefore providing 100% oxygen for even short periods of time can stop breathing altogether. As reptiles do not have a cough reflex (no diaphragm) and are relatively easy to intubate, conscious intubation of collapsed reptiles can be performed and IPPV administered for a short period.
If cardiac arrest occurs, intubation and intratracheal administration of epinephrine should be attempted. Reptiles can cope with a degree of hypoxia beyond that tolerated by mammals. IPPV after intubation is essential, although chest massage in the case of lizards and moving limbs into and out of the shell in chelonians may also be successful, aiding the pumping of air into and out of the lungs.
Many nutritional and husbandry diseases are common in reptiles, including metabolic bone disease and hypocalcaemic tetany in egg-bound mature lizards such as the green iguana. Calcium gluconate at 100 mg/kg may be administered in an emergency. Some of these lizards may fit, and diazepam or midazolam may be administered (see Table 24.1).
E for ECG
ECG deflections are generally small and rates, of course, slow. The rate is dependent on the external temperature the reptile is kept at.
In snakes, lizards and chelonians, metal crocodile clips with filed teeth may be used. Coupling gel or surgical spirit is required to optimise contact, and prolonged contact time is necessary to allow the gel to penetrate the keratin skin layer.
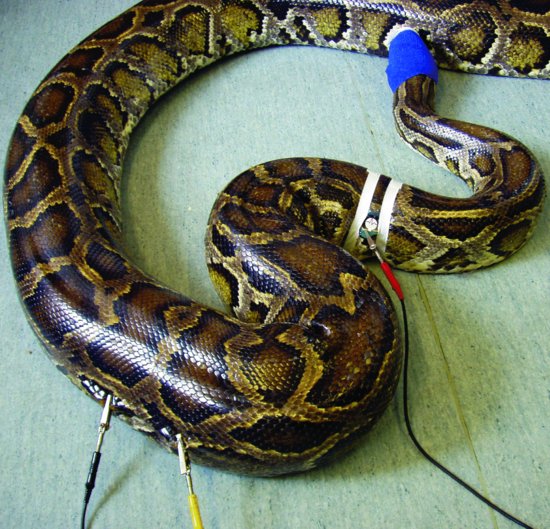
- Snakes: a base-apex reading is taken with electrodes placed two heart lengths cranial and caudal to the heart on the lateral aspect (Figure 24.4).
- Lizards: cranial leads are placed on the skin of the axilla, forelimb or neck, caudal leads on the crural or popliteal fold.
- Chelonians: cranial leads are placed on the cervical or axillary skin folds and caudal leads on the skin fold caudal to the hind limb.
Figure 24.4 Placement of ECG leads in a snake. (Reproduced with permission from Girling & Raiti, 2004, © BSAVA )
Reptile ECGs demonstrate a P wave, QRS complex and T wave pattern familiar to cardiologists. An additional SV waveform preceding the P wave representing depolarisation of the sinus venosus has been described but is a rarely seen.
Normal ECG findings include pleomorphism of the P wave, which may be single, peaked or biphasic. The QRS complex is often represented as a single R wave. Long repolarisation phases (longer QT and ST intervals) are present.
Allometric scaling may be used for the prediction of heart rate (HR = 33.4 × [Wtkg − 0.25], Sedgwick, 1991), assuming the reptile is maintained at its optimal body temperature.
Other useful drugs and techniques
Initial assessment of the collapsed reptile
An initial assessment should be made of the reptile patient before it is removed from its carry cage/box. This should focus on the following points:
Whilst examining the patient from a distance to ascertain if it is safe to handle, it is a good idea to question the owner about the husbandry of the reptile at home, i.e.,:
Detailed examination of the collapsed reptile
Manual restraint
This is covered in the section on anaesthesia and analgesia.
Detailed examination
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


