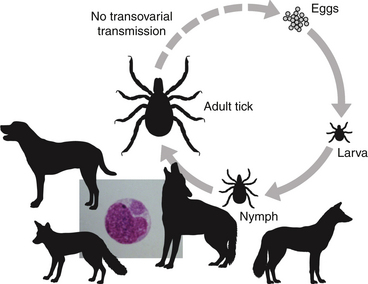
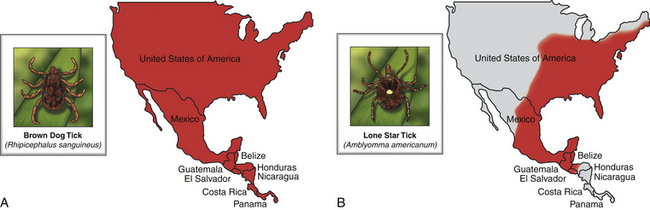
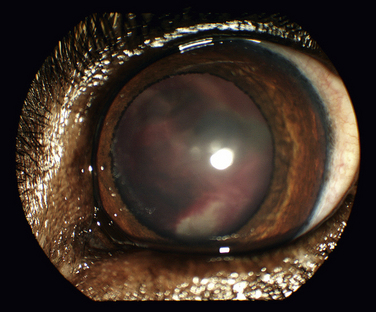
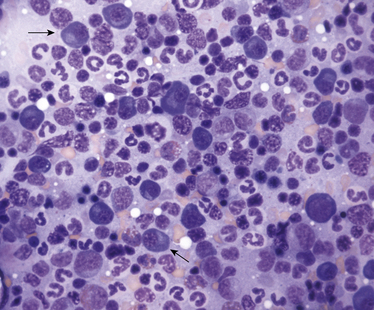
Chapter 28 The ehrlichioses are a group of tick-transmitted diseases caused by intracellular, gram-negative bacteria that include Ehrlichia canis, Ehrlichia ewingii, and Ehrlichia chaffeensis. An organism related to Ehrlichia ruminantium, the cause of heartwater disease in cattle, has also been detected in ill dogs from South Africa,5 and an organism that resembles E. muris has been detected in an ill dog and humans from the upper Midwest of the United States.4 These organisms form morulae (Latin for “mulberry”), a cluster of bacteria, within phagosomes of circulating leukocytes. Ehrlichia canis infects monocytes and causes canine monocytic ehrlichiosis (CME), one of the most important infectious diseases of domestic dogs that are exposed to ticks worldwide. Ehrlichia ewingii is an unculturable bacterium that infects granulocytes and causes canine granulocytic ehrlichiosis. Ehrlichia chaffeensis causes human monocytic ehrlichiosis; dogs are a proposed reservoir for this organism. The geographic distribution of each pathogen is generally restricted to that of their vectors and mammalian reservoir hosts. Organisms from the genus Ehrlichia are grouped within the family Anaplasmataceae. Also within this family are the bacteria Anaplasma platys and Anaplasma phagocytophilum, which cause canine thrombocytic and granulocytic anaplasmosis, respectively (see Chapter 29); and organisms belonging to the genera Neorickettsia (see Chapter 31). Rickettsia rickettsii, the cause of Rocky Mountain spotted fever (RMSF), and other spotted fever group rickettsiae belong to a separate family, the Rickettsiaceae (Chapter 30). The families Rickettsiaceae and Anaplasmataceae are phylogenetically related through the order Rickettsiales (Table 28-1). The recent availability of complete genome sequences for these organisms has helped to elucidate mechanisms of pathogenesis and host-pathogen interactions.5–7 E. canis is transmitted primarily by the brown dog tick (Rhipicephalus sanguineus), one of the most widely distributed ticks worldwide. Infection has been reported in dogs from Asia, Africa, Europe, and the Americas. Australia appears to be free of E. canis infection although occasionally seroreactivity to E. canis has been identified in dogs. The DNA of E. canis has been detected in other tick species, which include other Rhipicephalus species,8,9 Ixodes ricinus,10 Haemaphysalis spp. ticks,11 and Dermacentor spp. ticks11; experimental transmission has been accomplished with Dermacentor variabilis ticks.12 Different strains of E. canis exist that may vary in virulence. Although R. sanguineus is found throughout the United States, it prefers warm climates, and so disease is diagnosed most frequently in dogs living in the southeastern and southwestern states (Figure 28-1). In Europe, R. sanguineus is primarily found in Mediterranean regions, but its distribution appears to be moving northward, and CME has been reported in dogs that lack travel history as far north as the Netherlands.13 E. canis is the most common pathogen detected in ticks in Israel.8 Because of chronic, subclinical infection, dogs can be transported to non-endemic regions and subsequently develop disease years later. Tick larvae or nymphs acquire infection when they feed on infected dogs. Jackals, foxes, and possibly coyotes may also act as reservoir hosts. E. canis is transmitted transstadially (i.e., from larva to nymph to adult) within the tick (Figure 28-2).14 No clear age or sex predilection for CME exists, but German shepherds are reportedly more susceptible, and prognosis may be poorer in this breed. Cross-bred dogs may be less likely to develop disease.15 Although natural infection of cats with E. canis (or one or more closely related organisms) has been described in North and South America,16–18 clinical ehrlichiosis is rarely reported. FIGURE 28-1 A, Distribution of Rhipicephalus sanguineus, which transmits Ehrlichia canis, in the United States, Mexico and Central America. B, Distribution of Amblyomma americanum, which transmits Ehrlichia ewingii and Ehrlichia chaffeensis. The distribution of E. ewingii infections in dogs more closely matches that of A. americanum than the distribution of E. canis matches that of R. sanguineus (highest prevalence in the southern states). This is because of chronic E. canis infections that occur in dogs with travel histories to southern states, where the climate is warmer and R. sanguineus is more prevalent. The course of CME has been divided into acute, subclinical, and chronic phases, although in naturally infected dogs, these phases may not be readily distinguishable. Clinical signs of acute disease occur 8 to 20 days after infection. The organism multiplies by binary fission within vacuoles of mononuclear phagocytes; rupture of infected host cells leads to infection of new cells. Immune-mediated mechanisms are important in the pathogenesis of disease, and the presence of the spleen appears to contribute to disease severity.19 The clinical manifestations vary considerably among dogs, which may reflect factors such as E. canis strain variation, host immune response, stage of disease, and concurrent infections. Lethargy, inappetence, fever, and weight loss are most common. Replication of the organism in reticuloendothelial tissues is associated with generalized lymphadenopathy and splenomegaly. Ocular and nasal discharges, peripheral edema, and, less commonly, mucosal and cutaneous petechial and ecchymotic hemorrhages can also occur. Bleeding tendencies result from thrombocytopenia and platelet dysfunction,20 which may reflect immune-mediated platelet damage.21 Neurologic signs may result from meningeal inflammation or hemorrhage. Dogs can recover spontaneously from the acute phase within 2 to 4 weeks, after which time they may eliminate the infection or remain subclinically infected. Sequestration of organisms within the spleen may occur, and the organisms may evade the host immune system through antigenic variation.5 This subclinical phase may persist for months to years. Chronic CME develops in only some infected dogs. Factors that influence the development of chronic disease are unclear, but genetics may play a role. The presence of pancytopenia typifies the severe chronic form of ehrlichiosis, and results from hypoplasia of all bone marrow cells.22 Clinical signs range in severity and include lethargy, inappetence, bleeding tendencies, mucosal pallor, fever, weight loss, lymphadenopathy, splenomegaly, dyspnea, anterior uveitis, retinal hemorrhage and detachment, polyuria/polydipsia, and edema.15,22–24 Polymyositis occurs in some dogs, which can be manifested by diffuse muscle wasting and tetraparesis.25 Secondary opportunistic infections such as viral papillomatosis, protozoal infections, and bacterial urinary tract infections can also develop, although the precise underlying mechanism of immunosuppression, and how it relates to successful persistence of E. canis, has not yet been elucidated (see Figure 26-5).26,27 Marked granular lymphocytosis and bone marrow plasmacytosis may occur, sometimes accompanied by a monoclonal gammopathy, which may lead to misdiagnosis of lymphocytic leukemia or multiple myeloma, respectively. This has led to the recommendation that all dogs with well-differentiated lymphocytosis or otherwise unexplained monoclonal gammopathy be tested for E. canis infection.28 Protein-losing nephropathy may develop as a result of immune-complex glomerulonephritis. In reports of feline ehrlichiosis, clinical and laboratory findings have generally been similar to those in dogs; one cat had polyarthritis.16 Common physical examination findings in dogs with CME are lethargy, fever, peripheral lymphadenopathy, and splenomegaly. Ocular and nasal discharge, mucosal petechial hemorrhages, epistaxis, peripheral edema, and/or neurologic signs may be evident. Ocular abnormalities include anterior uveitis, hyphema, retinal hemorrhage, retinal detachment, and optic neuritis, with anterior uveitis being most common (Figure 28-3).24,29 Neurologic signs include twitching, ataxia, seizures, vestibular signs, hyperesthesia, and cranial nerve defects. Dogs with chronic ehrlichiosis may have thin body condition or diffuse muscle atrophy and mucosal pallor. Findings in cats with ehrlichiosis have been similar to those in dogs, and include lethargy, splenomegaly, lymphadenopathy, petechial hemorrhages, and retinal detachment.16,17 FIGURE 28-3 Retinal hemorrhage and detachment in a 4-year old female spayed Rhodesian ridgeback mix with canine monocytic ehrlichiosis. The dog also had moderate to severe, poorly regenerative, macrocytic hypochromic anemia (16.4% with a reticulocyte count of 49,000), lymphopenia (94 cells/µL), thrombocytopenia (26,000 platelets/µL), and large numbers of circulating nucleated red blood cells (75/100 WBC). Systolic blood pressure was within normal limits. (Courtesy of University of California, Davis Veterinary Ophthalmology Service.) Thrombocytopenia and occasionally mild leukopenia and a nonregenerative anemia occur 1 to 4 weeks after infection with E. canis. Mild thrombocytopenia, with increased mean platelet volume, may persist during the subclinical phase. Classically, dogs with chronic CME are pancytopenic, but more commonly, nonregenerative anemia and thrombocytopenia are noted. In some dogs, regenerative anemia or leukocytosis due to a neutrophilia and band neutrophils are present. Lymphopenia occurs in most affected dogs, but in some dogs, moderate to marked granular lymphocytosis (up to 17,000/µL) can occur. Normoblastosis, that can exceed 50 nucleated RBC per 100 WBC, may be present. In some dogs, morulae are visualized within circulating monocytes (Figure 28-2). The finding of morulae within monocytes using cytologic evaluation of blood smears is insensitive, especially in dogs with chronic infection, and does not distinguish between E. canis and E. chaffeensis infection. Use of buffy coat smears, thin smears of blood collected from the margin of the pinna, or splenic aspirates increases the sensitivity for detection of morulae. In one study, after careful searching, morulae were found in only 2 of 19 dogs with CME.22 Serum chemistry abnormalities in chronic ehrlichiosis include variable hypoalbuminemia, hyperglobulinemia, and elevated ALT and ALP activities. Most often the hyperglobulinemia is due to a polyclonal gammopathy.15 Monoclonal gammopathy can also develop. Less commonly, increases in serum urea nitrogen and creatinine concentrations are present.22 Transient proteinuria, with urine protein:creatinine ratios that exceed 20 (reference range, <1) have been reported in dogs with acute CME. This can resolve by 6 weeks after infection.30,31 Dogs with chronic CME may also have evidence of proteinuria. Pyuria, hematuria, and cylindruria may also be present. In addition to thrombocytopenia, coagulation abnormalities in dogs with CME include prolongation of the buccal mucosal bleeding time (BMBT), decreased platelet aggregation, and prolongation of the APTT.32,33 Dogs with central nervous system (CNS) involvement may have increased CSF protein concentrations and lymphocytic pleocytosis.24 Although rarely found, morulae may be detected in cells within the CSF.34 In dogs with chronic CME, bone marrow findings include hypoplasia or aplasia of all bone marrow elements, decreased iron stores and marrow plasmacytosis (Figure 28-4). Bone marrow mastocytosis has also been described.35 Myelofibrosis does not typically develop in chronic CME.36 Some dogs have normal or hypercellular marrows. FIGURE 28-4 Bone marrow plasmacytosis in an 8-year-old female spayed Labrador retriever with canine monocytic ehrlichiosis. Serum globulin was 12.1 g/dL (reference range, 2.3-4.4 g/dL), and serum protein electrophoresis revealed a polyclonal gammopathy. Plasma cells have a clock-faced nucleus and a clear area adjacent to the nucleus; two plasma cells are identified with arrows. Mild to moderate megakaryocyte hyperplasia, mild erythroid hypoplasia, and mild mature granular lymphocytosis were also present. Romanowsky stain. Available diagnostic assays for ehrlichiosis in dogs and cats are listed in Table 28-2. TABLE 28-2 Diagnostic Assays Available for Ehrlichiosis in Dogs and Cats CME, Canine monocytic ehrlichiosis; IFA, immunofluorescent antibody. Most often, the diagnosis of CME is made using serology, which may be performed using indirect immunofluorescent antibody (IFA) testing, ELISA technology, or Western blotting. Using IFA testing, which is considered the gold standard, antibodies can be detected between 7 and 28 days after initial infection. Dogs with acute ehrlichiosis may have false-negative test results if sufficient time has not elapsed for antibody production to occur. PCR assays may be helpful for diagnosis in this situation. A positive initial serum antibody titer may reflect previous exposure, and not necessarily ehrlichial disease. Retesting should be performed 2 to 3 weeks later to demonstrate seroconversion, and results of serology should be interpreted in light of a dog’s clinical signs and the results of testing for other potential causes of the dog’s illness. Dogs with chronic E. canis infection frequently have extremely high IFA titers, sometimes greater than 1:600,000, and these antibodies may persist in the face of treatment, suggesting persistence of the organism.22 Seroconversion does not generally occur in dogs with chronic disease, although antibody titers may decline in some dogs with treatment. High titers do not correlate with the severity of hyperglobulinemia, disease in general, or duration of illness. Because of variability of reporting between laboratories, there is no standard “cutoff” titer that is used to separate positive and negative results. Serologic cross-reactivity to other Ehrlichia species occurs, which includes E. ewingii and especially E. chaffeensis. Cross-reactivity to A. phagocytophilum antigens can occur to a lesser extent. In areas where other rickettsial agents are endemic, Western blotting has been used in an attempt to confirm that positive antibody titers on IFA are truly to E. canis antigens. However, Western blotting is laborious to perform and interpret and not routinely available, and when E. canis antigens are the target used, it may be difficult to distinguish between E. canis and E. chaffeensis infection.37 As a result, Western blotting has predominantly been used on a research basis. Whole-blood PCR assays for E. canis DNA is more sensitive for early diagnosis of CME than IFA or ELISA in dogs with acute disease. PCR assays are widely available for routine diagnosis of E. canis infection. Several laboratories offer panels that include PCR assays for a variety of different vector-borne pathogens. The results of these assays should be interpreted in light of a dog’s history, clinical signs, and the results of appropriate serologic assays; the last should be performed to support the results of PCR testing. PCR assays for E. canis may be performed on blood, lymph node aspirates, splenic aspirates, or bone marrow. Convalescent IFA or ELISA testing is much more sensitive than PCR assays for diagnosis of chronic CME.22,38,39 The sensitivity of PCR assays for diagnosis of CME when performed on bone marrow in dogs with chronic ehrlichiosis can range from 25% to 68%, depending on the laboratory.22 The use of PCR assays in the absence of serology is currently not suitable for screening potential blood donors for infection. PCR assays may be useful to confirm infection in the first week of illness, when serologic assays are often negative. Depending on the assay used, when positive, PCR can also be used to confirm the Ehrlichia species involved.
Ehrlichiosis
Ehrlichia canis Infection
Clinical Features
Physical Examination Findings
Diagnosis
Complete Blood Count
Serum Biochemical Tests
Urinalysis
Coagulation Profile
Cerebrospinal Fluid Analysis
Bone Marrow Analysis
Microbiologic Tests
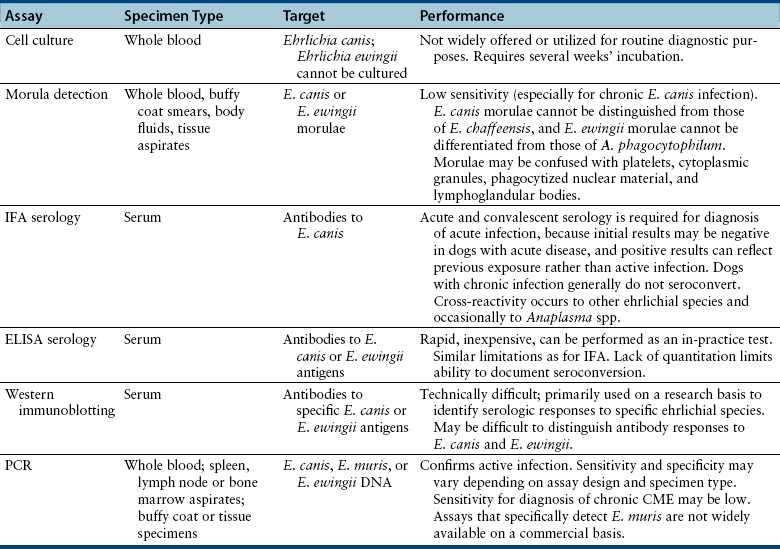
Serologic Diagnosis
Molecular Diagnosis Using the Polymerase Chain Reaction
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Ehrlichiosis
Only gold members can continue reading. Log In or Register to continue