CHAPTER 15 Effusions: Abdominal, Thoracic, and Pericardial
Abdominal viscera, thoracic viscera, and the heart are bathed in and lubricated by a small amount of fluid, which is essentially an ultrafiltrate of blood. The amount of lubricating fluid in the cavity is determined by the amount of fluid entering the cavity minus the amount exiting the cavity. Therefore the amount of fluid increases when more enters the cavity than is removed from it. Ectopic sources of fluid in the abdomen, such as rupture of the urinary bladder and hemorrhage into body cavities, are discussed in this chapter.
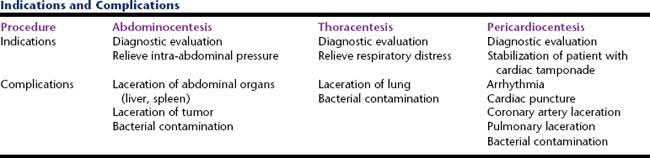
ABDOMINAL AND THORACIC EFFUSIONS
Most effusions are not noticed by pet owners until they become severe. Dogs and cats with pathologic thoracic effusions often show dyspnea as the most common clinical sign.1,2 Other clinical signs include a crouched, sternal recumbent position with extension of the head and neck; open-mouth breathing; tachypnea; and forceful abdominal respiration. Cyanosis may be present. With milder effusions, lethargy and lack of stamina may be the only clinical signs. Animals, especially cats, with mildly to moderately severe effusions often adapt by decreasing their activity, thus concealing their illness until it is severe. With chronic pleural effusion, dogs and cats may present with coughing as the only clinical sign.1
PERICARDIAL EFFUSIONS
Pericardial effusions are less common in cats than dogs. In cats, pericardial effusion is generally related to congestive heart failure (CHF) or feline infectious peritonitis (FIP), but can be caused by primary cardiac neoplasia such as lymphoma.3 The most common causes of pericardial effusion in dogs include cardiac neoplasia and idiopathic pericardial effusion (IPE).4,5 Hemangiosarcoma is the most common cardiac neoplasm reported, but other reported neoplasms include chemodectoma, lymphoma, and thyroid carcinoma.4
COLLECTION TECHNIQUES
Thoracentesis
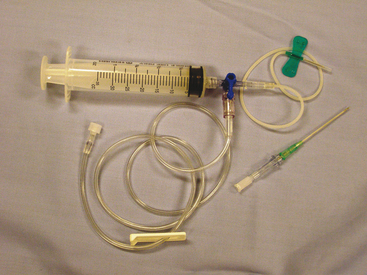
The animal is restrained in sternal recumbency or standing. The site of needle insertion is shaved and aseptically prepared. Tranquilization and/or local anesthesia are generally not necessary for collecting a small sample for analysis, but may be needed if a large amount of fluid must be drained from the chest. Large dogs may require a 1½-inch, 18- to 20-gauge needle or over-the- needle catheter, but a  -inch, 19- or 21-gauge. butterfly needle is preferred for cats and small dogs. The catheter unit allows the needle to be withdrawn after the catheter is introduced into the thoracic cavity, decreasing the chance of injury to intrathoracic organs.
-inch, 19- or 21-gauge. butterfly needle is preferred for cats and small dogs. The catheter unit allows the needle to be withdrawn after the catheter is introduced into the thoracic cavity, decreasing the chance of injury to intrathoracic organs.
The needle should be inserted next to the cranial surface of the rib to minimize the risk of lacerating the vessels on the rib’s caudal border. As long as the needle or catheter is below the fluid line, air will not be aspirated into the thoracic cavity. If only a single syringe of fluid is to be collected, the syringe may be attached to the catheter, the fluid aspirated, and the catheter withdrawn with the syringe attached. If a larger volume of fluid is to be removed or the syringe is to be repeatedly filled, extension tubing and a three-way stopcock should be attached (Figure 15-1).
Abdominocentesis
The ventral midline of the abdomen, 1 to 2 cm caudal to the umbilicus, is the usual site of needle insertion. This site avoids the falciform fat, which can readily block the needle barrel. The urinary bladder is emptied to help avoid accidental cystocentesis. The site of needle insertion is shaved and aseptically prepared. Neither local nor general anesthesia is usually needed. With the animal in lateral recumbency, a ventral midline puncture is made using a 1- to 1½-inch, 20- to 22-gauge needle, or a 20- to 16-gauge, 1½- to 2-inch plain or fenestrated over-the-needle catheter without the syringe attached. Free-flowing fluid should be collected into appropriate collection tubes. The needle can be rotated if fluid is not visible in the needle hub or a syringe can be attached and gentle negative pressure applied.6 If a previous surgical incision is present, the needle should be inserted at least 1.5 cm away from the site to avoid abdominal viscera that may have adhered to the abdominal wall in the area of the scar. The fluid is collected into an EDTA tube for cytologic examination, TP determination, and TNCC. A serum tube of fluid also is collected if any biochemical tests (e.g., creatinine, bilirubin) are to be performed, and fluid is collected in a culture-transport medium if culture is planned. To enhance fluid collection, abdominal compression may be applied when an over-the-needle catheter is used after the stylet has been removed, leaving only the catheter in the abdominal cavity. Although the catheter may kink, sufficient fluid can usually be collected for analysis.
If the technique just described fails to yield fluid, a four quadrant paracentesis or diagnostic peritoneal lavage (DPL) can be performed. In the four quadrant paracentesis the umbilicus serves as a central point and a paracentesis, as previously described, is performed in the right and left cranial and caudal quadrants.7 If DPL is performed, the animal is placed in dorsal recumbency, the area clipped and aseptically prepared, and a small 2-cm incision caudal to the umbilicus is made. Bleeders should be ligated to prevent blood contamination of the collected fluid. A peritoneal lavage catheter without the trocar is inserted into the abdominal cavity and directed caudally into the pelvis. A syringe is attached and gentle suction applied. If no fluid is obtained, warm sterile saline 20 ml/kg can be infused into the abdominal cavity. The patient is then rolled from side to side. The fluid can be collected via gravity drainage.6,7
Pericardiocentesis
Pericardiocentesis can be performed with the animal standing, in sternal or left lateral recumbency. Adequate restraint is needed to avoid cardiac puncture, coronary artery laceration, or pulmonary laceration. Sedation is used as necessary. Electrocardiogram (ECG) monitoring during pericardiocentesis is recommended, but not essential. Cardiac contact with the catheter or needle usually causes an arrhythmia. A large area of the right hemithorax from the third to the eighth rib is shaved and aseptically prepared. Local anesthesia including infiltration of the pleura with lidocaine can be used to minimize discomfort associated with pleura penetration. Puncture is generally between the fourth and fifth intercostal spaces at the costochondral junction. The needle is attached to a three-way stopcock, extension tubing, and syringe and gentle negative pressure is applied.5,8
A sample of the effusion should be collected in an EDTA tube to be used for a TNCC, packed cell volume (PCV), and TP determination, and cytologic examination. Other samples should be collected in a serum tube if any biochemical analyses are to be performed and in a culture-transport medium if culture is planned (Table 15-1).
SLIDE PREPARATION AND STAINING
The character of the fluid (turbid or clear) usually indicates the process(es) that may be occurring and the probable nucleated cell concentration. Clear, colorless fluids are usually transudates and of low cellularity. Although fluids of low cellularity are the most difficult to prepare, diagnostic-quality smears can be made from most low-cellularity fluids by using sediment or line smear techniques. Amber, clear to mildly opaque fluids are often modified transudates of low to moderate cellularity. Moderately to markedly opaque fluids, however, are usually exudates of moderate to very high cellularity. Slide preparation techniques for various types of fluids are briefly presented later. Chapter 1 contains a detailed discussion and illustrations.
Sediment smears should be made on all nonturbid fluid specimens. This is done by centrifuging the fluid for 5 minutes at 165 to 360 G. This can be achieved in a centrifuge with a radial arm length of 14.6 cm by centrifuging the fluid at 1000 to 1500 rpm. After centrifugation, nearly all of the supernatant is poured off, leaving only about 0.5 ml of fluid with the pellet in the bottom of the test tube. The supernatant may be used for refractometric TP determination and, if the supernatant was not collected from an EDTA tube, other chemical analyses. The pellet is then resuspended in the remaining 0.5 ml of fluid by gentle agitation, a drop of the suspension is placed on a glass slide, and a routine pull smear or squash prep is made (see Chapter 1). The smear is air-dried and then stained with any hematologic stain.
If it is not possible to centrifuge the fluid, line smears should be made from fresh, well-mixed fluid. Also, line smears are useful when making smears from the sediment of very low-cellularity fluids. Line smears are made by placing a drop of fluid on a glass slide and starting to make a pull smear. Instead of continuing until the fluid makes a feathered edge, go only a short distance, stop, and lift the pull slide directly up. Cells concentrate in the fluid following the pull slide, creating a line of increased cellularity where the pull slide is lifted (see Chapter 1).
When submitting samples to an outside laboratory for cytologic examination, it is best to request instructions for sample preparation from the person who will be examining the preparations. However, the following instructions generally suffice. Include direct smears and stained (optional) and unstained air-dried smears and prepared as described previously, along with an EDTA tube of the fluid. If pre-made, air-dried slides are not submitted and the fluid is delayed, the cellular constituents will begin to deteriorate, become hypersegmented and pyknotic, and cell morphology will be difficult if not impossible to evaluate. Even if the cells in the EDTA tube have become pyknotic, a TNCC and refractometric TP determination can be performed.10
Many acceptable stains are available. Diff-Quik (Harleco, Gibbstown, NJ) is one of the easiest to use. It stains cellular elements well and has little precipitate to confuse with bacteria. Some other commonly used hematologic stains are Wright’s stain and new methylene blue (NMB). Gram stain and acid-fast procedures may be used to classify bacteria, but are much less sensitive than hematologic stains for finding bacteria. Also, care must be given to procedure when performing a Gram stain because the cellular elements, proteins, and other constituents in septic fluids can affect the staining.10
LABORATORY DATA
Cell Counts and Counting Techniques
A TNCC, which is a count of all nucleated cells present in the fluid, may be done by automated or manual methods. The automated cell counters may count debris, so only relatively clear fluids should be used. The Unopette (Becton Dickinson Rutherford, NJ) procedure is a good in-house method and is performed the same as for peripheral blood leukocyte counts. Cell clumping, cell fragmentation, and noncellular debris can cause counting errors with both the automated and manual techniques.10
Red blood cell (RBC) counts offer little if any additional information over visual assessment and a PCV measurement on the effusion. Therefore RBC counts are seldom performed unless the TNCC is done on an automated instrument that automatically counts RBCs. Noting the presence of RBCs in effusions is important because they indicate blood contamination of the fluid during collection, hemorrhage into the cavity, or increased capillary permeability with diapedesis of RBCs into the cavity.10
Total Protein Measurement and Techniques
Effusion fluid protein concentration is used, with the TNCC, to classify effusions (transudates, modified transudates, exudates) and to estimate the severity of inflammation. The TP content may be determined biochemically or estimated by refractometry. The method of choice for determining TP concentration in peritoneal, pericardial, and pleural fluid is refractometry.11 If the fluid is opaque, it is best to determine the refractive index of the supernatant after centrifuging the fluid. Otherwise, refraction of light by suspended nonprotein particles (i.e., lipoproteins, urea, cholesterol, and glucose) may result in an erroneously high TP reading.10,11 Chylous or lipemic fluids often do not separate sufficiently to allow TP to be estimated by refractometry or chemical methods.
Other Tests
The pH testing of pericardial fluid initially was thought to assist in differentiating neoplastic from nonneoplastic effusions.12 A later study conducted on 42 client-owned dogs found the pH of pericardial fluid from dogs with neoplasia was higher than pericardial fluid from dogs with nonneoplastic etiologies; however, there was significant overlap between the two groups. Because of this overlap it was determined the use of pH testing is not useful in differentiating neoplastic from nonneoplastic conditions.13 In a more recent study by de Laforcade et al,14 pericardial effusion samples were collected on 41 client-owned dogs with pericardial effusions for biochemical analysis. Results of pH testing of the effusion fluid differed from previous studies in that the pH of dogs with neoplasia was lower than nonneoplastic conditions; however, again the degree of overlap of the two populations limited the usage of pH testing to differentiate neoplastic from nonneoplastic conditions.14 The differences in pH values between the studies may have been due to differences in methodologies used in determining the pH of the effusions.
MICROBIOLOGIC CULTURES
Not all effusions need to be cultured (e.g., true transudates). If cytologic evaluation of the fluid suggests infection, the fluid should be cultured for both aerobic and anaerobic bacteria. Fluid samples for aerobic and anaerobic cultures can be collected into a syringe, taking care to exclude air from the sample. The needle should be capped and the syringe containing the sample taken immediately to the laboratory for culture. For optimal results or if a delay is anticipated, the fluid should be placed in a transport system that supports both aerobic and anaerobic bacteria. Chapter 1 contains a further discussion of culturing.
CELLS AND STRUCTURES SEEN IN EFFUSIONS
Neutrophils
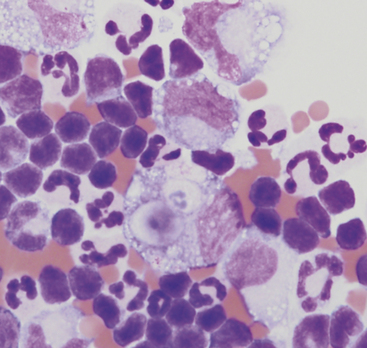
Degenerate neutrophils are neutrophils that have undergone hydropic degeneration. This is a morphologic change that occurs in tissue or effusions because bacterial toxins alter cell membrane permeability. This allows water to diffuse into the cell and through the nuclear pores, causing the nucleus to swell, fill more of the cytoplasm, and stain homogeneously eosinophilic. This swollen, loose, homogeneous eosinophilic nuclear chromatin pattern characterizes the degenerate neutrophil (Figure 15-2). Although all cell types are exposed to the same toxin, degenerative change is evaluated only in neutrophils.
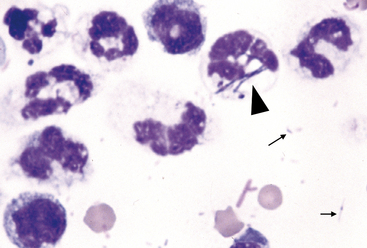
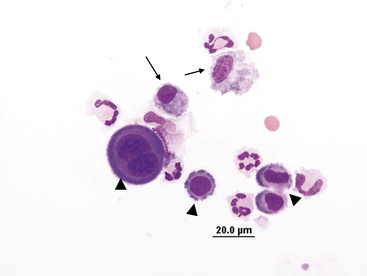
Nondegenerate neutrophils, such as peripheral blood neutrophils, are those with tightly clumped, basophilic nuclear chromatin (Figure 15-3). Some neutrophils in effusions may be hypersegmented. Hypersegmentation is an age-related change; the nuclear chromatin condenses and eventually breaks into round, tightly clumped spheres (pyknosis) (Figure 15-4). These aged neutrophils are often seen phagocytized by macrophages (cytophagia) (Figure 15-5). The presence of nondegenerate neutrophils suggests that the fluid is not septic; however, bacteria that are not strong toxin producers, such as Actinomyces spp., may be associated with nondegenerate neutrophils. Also, nonbacterial infectious agents (e.g., Ehrlichia and Toxoplasma spp. and various fungi) may be associated with nondegenerate neutrophils.
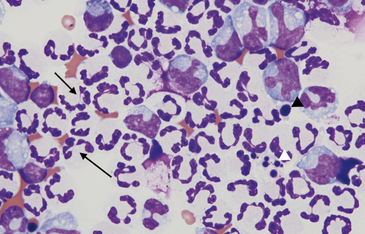
Figure 15-4 Abdominal fluid from same cat as Figure 15-3. Numerous nondegenerate neutrophils. Some neutrophils are hypersegmented with only thin chromatin strands connecting the segments (small arrows). Pyknotic nuclei (arrowheads), are also present.
Effusions may also contain toxic neutrophils. Toxic changes (e.g., Dohle bodies, toxic granulation, diffuse cytoplasmic basophilia, foamy cytoplasm) develop in the bone marrow in response to accelerated granulopoiesis due to inflammation. Toxic neutrophils in the peripheral blood migrate into the body cavity and are observed in effusions of the cavity.10 Although foamy cytoplasm is considered a toxic change, cytoplasmic vacuolation may be seen in neutrophils of peritoneal/thoracic fluid smears because of age-related change or EDTA-induced artifact.
Mesothelial/Macrophage-Type Cells
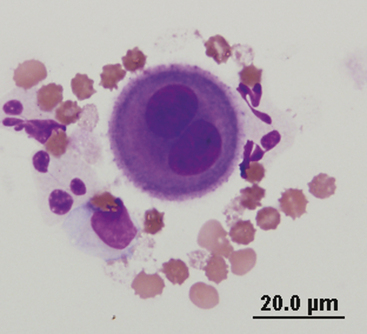
Mesothelial cells line the pleural, peritoneal, and pericardial cavities as well as visceral surfaces, and are present in variable numbers in most effusions (Figures 15-6 and 15-7). They are large cells that may be present singly or in clusters or rafts. They generally contain a single round to oval nucleus but may be multinucleated. The nuclear chromatin has a fine reticular pattern. Nucleoli may be present in activated mesothelial cells. Activated mesothelial cells may have morphologic characteristics similar to cells from malignant neoplasms, but should not be confused with neoplastic cells. Mesotheliomas are rare in small animals and difficult to diagnose cytologically. Their cytoplasm is slightly basophilic and may contain phagocytic debris, because activated mesothelial cells may become phagocytic. A blue or red corona may be present around nucleated cells within effusions, especially mesothelial cells, as an artifact of slow drying and is of no diagnostic significance.
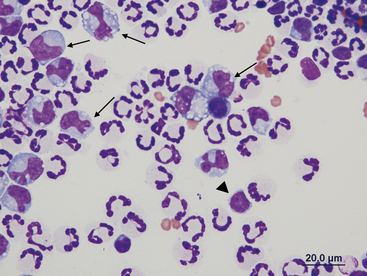
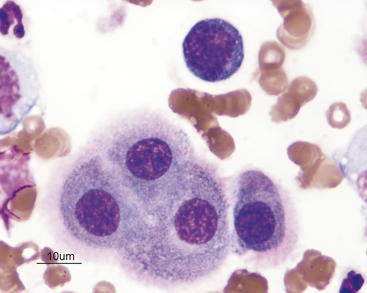
Peritoneal macrophages generally have a single oval to bean-shaped nucleus; however, multinucleation and variable nuclear shape may be present (Figures 15-8 and 15-9). Their nuclear chromatin is lacy and their cytoplasm is frequently vacuolated and may contain phagocytic debris.
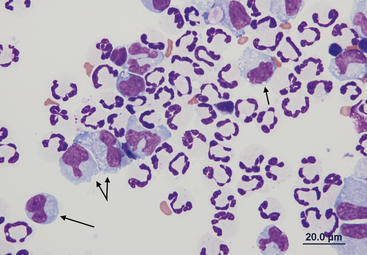
Figure 15-9 Numerous nondegenerate neutrophils and macrophages with variable nuclear morphologies (arrows).
Lymphocytes
Lymphocytes are present in many effusions and may be the predominant cell type in chylous and lymphomatous effusions. With lymphoma, neoplastic lymphocytes frequently—but not always—exfoliate into the fluid and are present in large numbers. Chylous effusions primarily consist of small lymphocytes (Figure 15-10), whereas lymphomatous effusions primarily consist of lymphoblasts (Figures 15-11 and 15-12). The small lymphocytes seen in chylous effusions have a small amount of clear to blue cytoplasm, an oval to bean-shaped nucleus, clumpy nuclear chromatin, and no visible nucleoli. These cells are typically smaller than neutrophils. Reactive lymphocytes (Figure 15-13) may be seen in inflammatory effusions. These lymphocytes are slightly larger than small lymphocytes, have a small to moderate amount of very blue cytoplasm, and may become plasmacytoid.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree