Philip J. Johnson
Dyslipidemias
From the equine medical perspective, the term dyslipidemia refers to clinical situations in which an increase in plasma triglyceride (TG) concentration above the reference range (6 to 54 mg/dL [0.07 to 0.61 mmol/L]) has occurred, a condition also known as hypertriglyceridemia. Reported reference ranges for plasma TG in donkeys and ponies tend to be higher (up to 290 mg/dL). Hypertriglyceridemia represents a normal physiologic response to the need for mobilization of body fat reserves, typically in response to a negative energy balance, physiologic stress, or both.
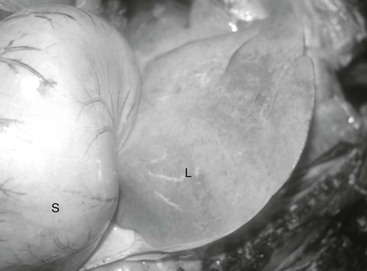
The term lipemia is used if plasma turbidity (lactescence) is evident on visual examination of a blood sample (Figure 137-1). It is important to realize that lipemia does not refer to a clinical condition. The term hyperlipidemia is used to describe mild hypertriglyceridemia (54 to 500 mg/dL) in the absence of lipemia. Greater elevations of plasma TG concentration (>500 mg/dL) are described by the term hyperlipemia and may be associated with lipemia, infiltration of organs (especially the liver and kidneys) by lipid, and clinical disease, especially hepatic lipidosis. Miniature Horses, ponies, and donkeys are especially prone to profound lipid mobilization (hyperlipemia) and significant clinical consequences because they often concomitantly have insulin resistance (IR). Insulin regulates lipid mobilization in the body, and an absence of insulin influence, as a consequence of either reduced pancreatic secretion or peripheral IR, is a major risk factor for development of hyperlipemia. Recently, “marked hypertriglyceridemia” in the absence of lipemia or organ dysfunction has also been identified in large-breed horses during hospitalization. The importance of severe hypertriglyceridemia in large-breed horses is not clear, but the condition should be considered in hospitalized horses facing either a negative energy balance or physiologic stress. The term primary or idiopathic hepatic lipidosis is used if another disease state cannot be identified. When an additional disease state causes anorexia and precedes hepatic lipidosis, the hepatic lipidosis is defined as secondary.
Insulin plays a very important role in the regulation of circulating TG because, in the healthy state, it both inhibits hormone-sensitive lipase (HSL) in adipose tissues and stimulates lipoprotein lipase (LPL) in peripheral tissues that obtain energy from circulating TG. Hormone-sensitive lipase is one of two enzymes, along with adipose triglyceride lipase, that serve to mobilize TG from adipose tissues, a process known as lipolysis or TG hydrolysis: TG is converted to free fatty acids (FFAs) and glycerol, both of which enter the circulation. Although lipolysis may be stimulated by several lipolytic hormones (glucagon, epinephrine, norepinephrine, cortisol, ghrelin, growth hormone, and testosterone), at times of “need,” such as during periods of negative energy balance and physiologic stress, insulin acts alone to inhibit lipolysis and promote TG storage by TG esterification or lipogenesis.
During negative energy balance caused by fasting and starvation or periods of illness-associated anorexia, the secretion of glucagon is increased at a time when pancreatic insulin secretion is inhibited so that normoglycemia can be maintained. Although glucose is mobilized through stimulated glycogenolysis and gluconeogenesis, glycogen reserves are limited, and hypoglycemia tends to develop. Hypoglycemia provokes the release of lipolytic hormones that strive to maintain normoglycemia (Somogyi effect). Energy requirements are therefore met through mobilization of both adipose tissues (physiologic hypertriglyceridemia) and amino acids, and the horse enters a net catabolic state. Amino acids are derived from muscle proteins and may be converted to glucose in the liver. Moreover, if physiologic stress is also present, stress hormone release further stimulates lipolysis and protein catabolism: cortisol is inhibitory to both the secretion and peripheral action of insulin, and catecholamines directly stimulate lipolysis in adipose tissues.
Accumulation of mobilized lipids by nonadipose tissues may cause organ failure (lipotoxicity). For example, in skeletal muscle, which is the major insulin-mediated glucose sink, excessive intramyocellular lipid inhibits insulin sensitivity. Moreover, accretion of lipid by renal tubular cells leads to azotemia and renal failure. Azotemia is an independent risk factor for hyperlipemia because azotemia both inhibits LPL and is associated with reduced urinary lipid clearance consequent to the lowered glomerular filtration rate.
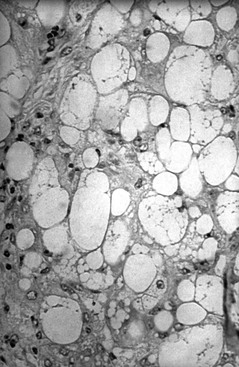
Normally, circulating FFAs are taken up by tissues and converted to energy through β-oxidation. In the presence of accelerated lipolysis and in the absence of insulin or its effect, increased HSL action can lead to excessive FFA release from adipose tissue repositories. In this circumstance, FFAs are removed from the circulation by hepatocytes and may be used for β-oxidation, converted into ketone bodies, or converted to TG. Hepatocytes repackage TG, which is insoluble in plasma water and circulates in plasma in the form of lipoproteins, and export it as very-low-density lipoprotein (VLDL) for distribution to peripheral tissues. Excessive FFA mobilization overwhelms hepatocyte processing to VLDL, leading to hepatic lipidosis and liver dysfunction (Figures 137-2 and 137-3). Cells in peripheral tissues use LPL for purposes of TG extraction from circulating VLDL. The activity of LPL is stimulated by both insulin and glucose-dependent insulinotropic polypeptide, an incretin hormone released by the gastrointestinal tract following consumption of carbohydrates and fat. In equid species, hyperlipemia is characterized by severely high plasma VLDL concentration. The VLDL particles produced in excess by the liver during hyperlipemia are unusual (compared with normal VLDL particles) in that their mean diameter is 44% greater and their structural apolipoprotein is apoB-48 instead of apoB-100 (this modification is presumed to facilitate export of a relatively greater mass of TG).
To a greater and greater extent under contemporary management practices, horses and ponies are fed energy-dense rations characterized by high sugar and starch content that far exceed the daily energy requirements. Coupled with physical inactivity, these practices have made obesity an increasingly common problem. Physical inactivity and obesity represent inherent risk factors for worsening IR and therefore predispose to hyperlipemia in affected individuals.
Risk Factors and Predispositions for Hyperlipemia
The principal risk factors for clinical hyperlipemia and organ failure include a negative energy balance, lack of insulin or IR, stress, and azotemia. Negative energy balance may result from overt starvation, eating poor-quality forage, fasting during the management of alimentary tract disease, aggressive dietary restriction to treat obesity, or the appetite-depressing effects of illness or administered medications. Negative energy balance especially increases the risk for hyperlipemia during late gestation and lactation in Shetland Ponies, Miniature Horses, and donkeys, breeds that are inherently insulin resistant. However, hyperlipemia and hepatic lipidosis can also occur in large-breed horses such as Quarter Horses, Tennessee Walking Horses, and Paso Finos.
Aside from breed predisposition, other factors that may act independently to promote IR and increase risk for hyperlipemia include obesity; pregnancy and lactation; inclement, especially cold, weather; inflammatory diseases; stress; pain; certain drugs (corticosteroids, thiazide diuretics, and phenothiazine tranquilizers); advancing age; lack of exercise; and endocrine conditions associated with IR, such as equine metabolic syndrome and pituitary pars intermedia dysfunction. Other commonly reported primary conditions that are sometimes complicated by hyperlipemia include enterocolitis, endotoxemia, endoparasitism, neoplasia, altered management, and neonatal septicemia. Inflammatory conditions increase the risk for hyperlipemia by reducing food intake and elevating concentrations of circulating proinflammatory cytokines such as tumor necrosis factor-α, which is an inhibitor of insulin action.