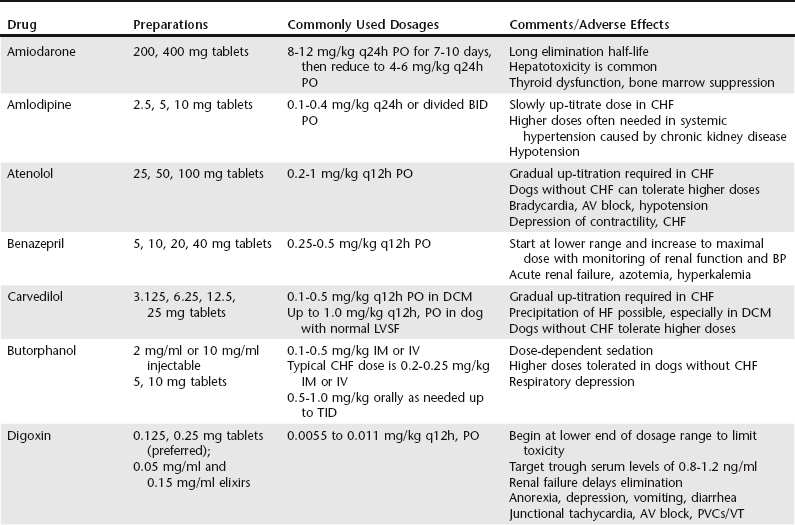
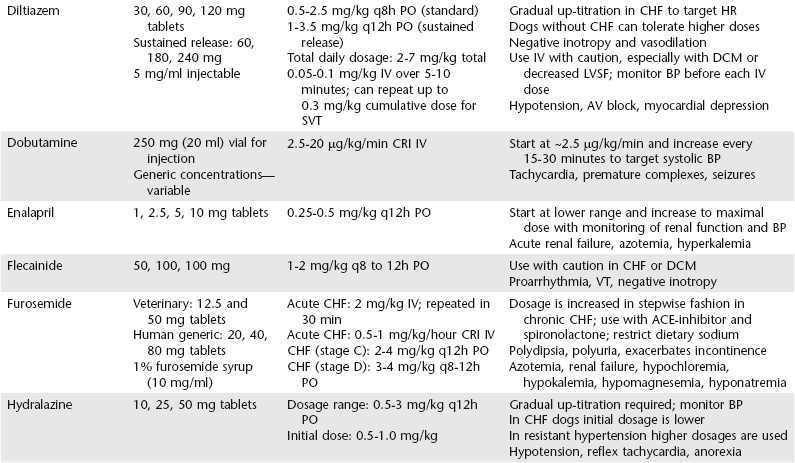
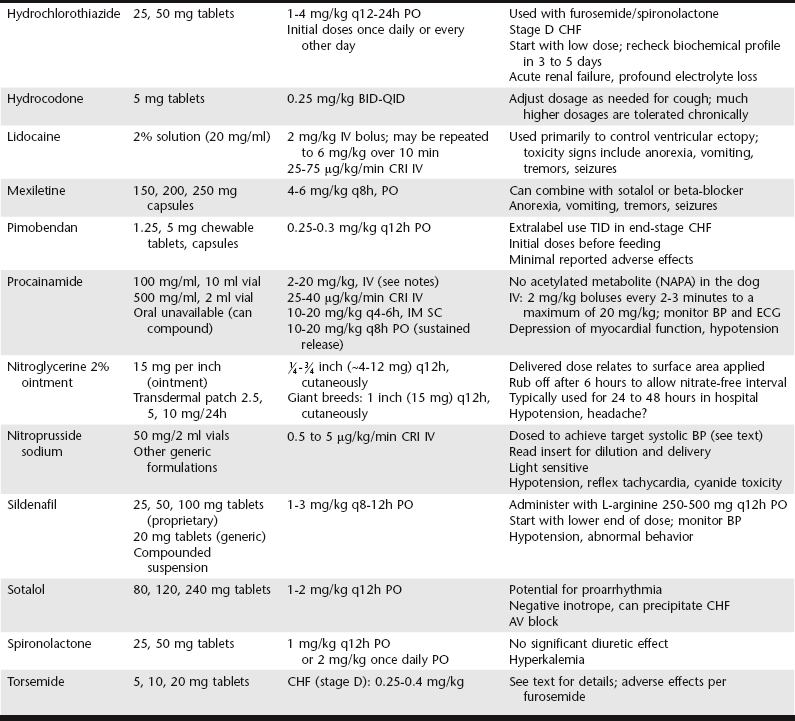
Chapter 175 Therapy for canine heart failure (HF) requires careful orchestration of a multiple-drug treatment regimen. Cardiovascular drugs are potent in their relief of clinical signs and can be life prolonging, but these compounds also can injure the patient. In this chapter we consider the general classification, mechanism of action, indications, uses, and adverse effects of drugs used for treatment of canine HF. The coordinated use of these drugs and some of the clinical trial data indicating their benefit are discussed next in Chapter 176. The reader also is directed to the Appendix for a listing of alternative dosages. The dosages summarized in Table 175-1 focus exclusively on dogs; dosages for cats can be found in Chapters 173 and 180. One of the authors (BK) also has developed an on-line dosage calculator that veterinarians and veterinary technicians may find useful (see www.cardiologycarenetwork.com/network/dosage_calc.php). The number of drugs capable of affecting heart and vascular functions is considerable (Box 175-1), and the pharmacologic effects of drugs used in treatment of HF can become confusing. Some treatments for congestive heart failure (CHF) affect ventricular pumping (inotropes), whereas others reduce venous pressures and ventricular preload (diuretics and venodilators), improving or preventing signs of congestion. Drugs with arterial vasodilator effects decrease blood pressure (BP), ventricular afterload, and mitral regurgitant volume, with the consequence of increasing forward stroke volume leaving the ventricle. Some agents demonstrate very rapid hemodynamic effects (e.g., intravenously administered diuretics, nitrovasodilators, and catecholamines). Others confer benefits by modulating the chronically activated neurohormonal, inflammatory, and profibrotic mediators of CHF (these include the “cardioprotective” drugs such as the angiotensin-converting enzyme [ACE] inhibitors, β-adrenergic blockers, and aldosterone receptor blockers). The clinician should be mindful of the clinical pharmacology of these compounds (Brunton et al, 2010; Gordon and Kittleson, 2008; Opie and Gersh, 2013) and also appreciate that many drugs we use in veterinary practice are prescribed in an extralabel manner. For example, most of the vasodilator drugs and all of the antiarrhythmic drugs mentioned in this chapter are approved for human use and administered off label to dogs based on current standards of care. Although this chapter offers an overview of the important drugs used to manage HF in dogs, the reader is directed to comprehensive textbooks in the reference list for a fuller recounting. The ABCD stages of HF are described fully in the next chapter, but for reference, stage C represents CHF that is manageable with standard four-drug therapy and dosages and stage D represents refractory CHF that requires additional therapies and higher dosages of the standard-treatment drugs. In this context, standard therapy for CHF in the dog is furosemide, an ACE inhibitor, pimobendan, and spironolactone. Diuretics are administered to cardiac patients in acute situations for mobilization of edema and in chronic HF to prevent ongoing sodium and water retention. Low dosages also may be useful (when combined with an ACE inhibitor) in the control of cough related to left bronchial compression when it occurs before the onset of overt CHF. When diuretics are prescribed for long-term use, dietary sodium intake should be restricted (see Chapter 168) and an ACE inhibitor and aldosterone receptor blocker (spironolactone) coadministered. Oral maintenance dosages of furosemide can be initiated once the dog is in clinically stable condition and ready to be released from the hospital. The usual oral dosage is 2 to 4 mg/kg two times daily. The frequency can be increased to three times daily and the dosage up-titrated to as high as 12 mg/kg daily in refractory cases of CHF provided renal function and electrolyte status are monitored. When fluid retention becomes unresponsive to standard dosages of furosemide (stage D CHF), other diuretic options should be considered, including flexible subcutaneous dosing of furosemide or the addition of another diuretic such as torsemide or hydrochlorothiazide (see Chapter 176 for details). Torsemide ostensibly is more potent than furosemide in this setting and may be considered at approximately Adverse effects of loop diuretics include polydipsia, polyuria, reduction in BP, plasma volume depletion, (prerenal) azotemia, and depletion of electrolytes, especially chloride, potassium, and magnesium. Monotherapy with a loop diuretic activates the renin-angiotensin-aldosterone system; accordingly, long-term diuretic therapy should incorporate an ACE inhibitor (and optimally spironolactone) as part of the treatment plan. Clients should be instructed not to administer the drug at bedtime to avoid urinary accidents, and they should never restrict water (except in rare circumstances of profound hyponatremia). Mild azotemia is not a reason to discontinue diuretic therapy, but moderate to severe azotemia should prompt dosage reductions (see Chapter 176 for a detailed discussion of this issue). Potassium supplements are needed infrequently in dogs receiving combined therapy with furosemide, spironolactone, and enalapril because the latter two drugs “spare” potassium by reducing urinary losses. Ototoxicity and renal failure are potential adverse effects, especially when aminoglycosides are coadministered. ACE inhibitors reduce serum aldosterone concentration and limit sodium retention and potassium loss in the urine. Additionally, ACE inhibitors protect cardiac muscle, renal tissues, and blood vessels from RAAS-induced injury while down-regulating the sympathetic nervous system. Vasodilation associated with ACE inhibition is not as dramatic as that observed with calcium channel blockers or intravenous nitrates so the overall effect on arterial BP is modest, especially in the treatment of dogs with systemic hypertension. The published dosage ranges for enalapril and benazepril vary considerably from as low as 0.25 mg/kg once or twice daily to as high as 0.5 mg/kg twice daily. The authors generally start with enalapril at approximately 0.25 mg/kg q12h PO and double the dosage to approximately 0.5 mg/kg q12h PO at the time of first reevaluation as long as arterial BP and renal function are acceptable (see Chapter 176).
Drugs for Treatment of Heart Failure in Dogs
Diuretics
Furosemide and Torsemide
 of the furosemide milligram dosage. It can be substituted for one or more daily doses of furosemide.
of the furosemide milligram dosage. It can be substituted for one or more daily doses of furosemide.
Angiotensin-Converting Enzyme Inhibitors and Other Vasodilators
Angiotensin-Converting Enzyme Inhibitors
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree