Chapter 12 Drugs for the Treatment of Protozoal Infections
This chapter does not include the protozoa that appear only sporadically in the veterinary literature (Balantidium, Pentatrichomonas, Entamoeba, Hammondia, Besnoitia, and Sarcocystis spp.). These organisms are not adequately documented as pathogens of dogs and cats and thus require no therapy. Also excluded are selected protozoal pathogens that are partially characterized but have no effective treatment available. Textbooks of parasitology or infectious disease should be consulted for a complete discussion of these sporadic, spurious, or untreatable pathogens.1,2
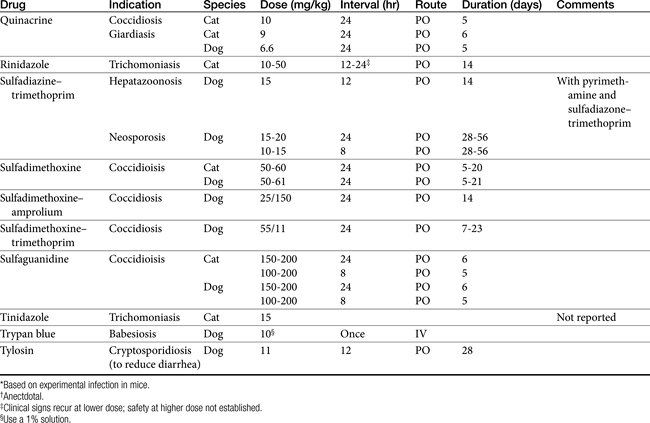
Therapy of protozoal infections, which are often zoonotic, must include use of therapeutic agents along with supportive therapy and proper hygiene and husbandry to clean up the environment and prevent spread to other animals and people. No therapeutic agent, no matter how safe or effective, can be expected to treat these diseases without supportive therapy and hygiene. Table 12-1 lists the therapeutic agents discussed in the text. These drugs are discussed below as well as in specific chapters.
Common Enteric Coccidiosis
Biology
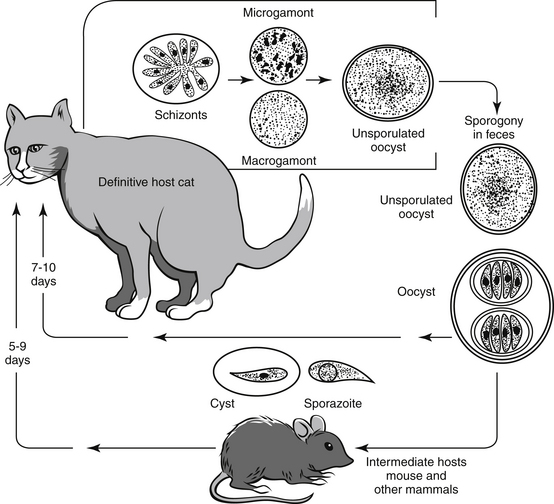
Coccidia have life cycles that are more complex than other infectious agents (Figure 12-1). Each life cycle includes both sexual and asexual phases. This is important to remember because the therapeutic agents used to treat and control coccidia are primarily effective against the asexual stage of the life cycle.
The repeated intracellular invasion of enterocytes and subsequent rupture can produce substantial pathology to the gut, especially if the infected host is young, weak, malnourished, or stressed. Normal animals, in otherwise good health, usually experience coccidial infection followed by an effective immune response that limits and eliminates the infection without therapeutic intervention. Most clinicians prefer to intervene when coccidia are identified in a fecal floatation. Therapy is usually successful in eliminating the coccidial oocysts, although it is not known how many of these animals would have spontaneously cleared the infection without intervention.
Treatment
Sulfas and Potentiated Sulfas
Use of sulfonamides is the treatment of choice for small animal coccidia as well as a number of other protozoal organisms. Unfortunately there is a paucity of research information to support their efficacy. Two pivotal studies on sulfamethoxine and sulfaguanidine against coccidia support their utility; however, these two agents are no longer available in the United States.3,4 Clinicians have empirically substituted more readily available sulfonamides and enjoyed apparent clinical success.5 Currently there is one simple sulfa and three potentiated sulfas that are commonly used in the United States: sulfadimethoxine (Albon), sulfadimethoxine with ormetoprim (Primor), sulfadiazine with trimethoprim (Di-Trim, Tribrissen), and sulfamethoxazole with trimethoprim (Bactrim, Septra).
Sulfonamide Chemistry and Mechanism of Action
The sulfonamides are discussed in more depth in Chapter 7. Each is a structural analog of para-aminobenzoic acid that competitively inhibits the dihydropterate synthetase step in the synthesis of folic acid, which is required for synthesis of RNA and DNA. Inhibition by sulfas impairs protein synthesis, metabolism, and growth of the pathogen. A vast array of sulfa agents have been created and described; all but a few have been lost in the sands of time. The important differences among these agents involve their solubility, duration of action, and activity against key pathogens. Fortunately, the three sulfas included in this discussion demonstrate acceptable performance in all three categories; solubility is adequate, they are given once or twice daily, and they have a reasonably broad spectrum of action. The sulfa drugs are primarily effective against the schizont stages of coccidian; thus prolonged treatment may be required for the drug to effectively block the life cycle.
Potentiator Chemistry and Mechanism of Action
The diaminopyrimidine potentiators (trimethoprim and ormetoprim) act in concert with sulfonamides by blocking the next step (dihydrofolate reductase) in folic acid synthesis. Chemically the diaminopyrimidines are related to pyrimethamine, which has antimalarial properties. The agents are highly selective inhibitors of dihydrofolate reductase. This sequential blockade produces significant potentiation of activity. It is a classic case of drug potentiation.
Toxicity and Adverse Effects
The long history of sulfa use in veterinary medicine has resulted in a wide array of toxic and idiosyncratic reactions in animals. Historically, the most common and most avoidable reactions result from crystallization in the urinary tract with secondary crystalluria, hematuria, and urinary obstruction. Recent reviews in human medicine indicate that the improved solubility of the modern preparations has decreased the risk of crystalluria; nevertheless, it is still prudent to ensure adequate water intake and proper hydration during sulfa therapy.6 The human-medicine literature also suggests that the sulfonamides may be directly nephrotoxic.6 Hematopoietic disorders (thrombocytopenia and leukopenia) have also been reported as a result of sulfa therapy. Sulfaquinoxaline especially has been associated with hypothrombinemia, hemorrhage, and death in puppies receiving therapy for coccidia.7
Sulfadimethoxine
Sulfadimethoxine is a rapidly absorbed, long-acting sulfonamide. It is not acetylated in the dog and is excreted unchanged in the urine. It is approved for treatment of coccidiosis in dogs and cats. It has a wide margin of safety; dogs given multiple oral doses of 160 mg/kg by mouth daily for 13 weeks showed no signs of toxicity.8
Sulfadimethoxine with Ormetoprim
Sulfadimethoxine with ormetoprim is the most recently approved potentiated sulfonamide. It constitutes a rational combination that potentiates the action of both drugs by blocking two sequential steps in the synthesis of folic acid. Ormetoprim is a diaminopyrimidine potentiator with very low mammalian toxicity. The available tablets contain 100/20, 200/40, 500/100, or 1000/200 mg sulfadimethoxine/mg ormetoprim, respectively (Primor). The tablets are designated by the total weight of active ingredient in each tablet; thus Primor 120 contains 100 mg of sulfadimethoxine and 20 mg of ormetoprim. The approved starting dose is 55 mg/kg orally on the first day of treatment and then 27.5 mg/kg orally once per day for 14 to 21 days. Treatment should not extend beyond 21 days.8
It is interesting to note that the only recent controlled study of coccidiosis therapy for dogs was conducted with this drug combination. In that study, 32.5 mg/kg or 66 mg/kg was given continuously in the food for 23 days, subsequent to experimental oocyst infection. The higher dose of 66 mg/kg provided better results and did not produce any adverse reactions.9
Sulfadiazine or Sulfamethoxazole with Trimethoprim
Sulfadiazine with trimethoprim is the potentiated sulfa with the most years of actual use in veterinary medicine. For many years it was the only potentiated sulfa approved for use in animals. Trimethoprim is a diaminopyrimidine potentiator with very low mammalian toxicity. The available tablets contain 25/5, 100/20, 400/80, or 800/160 mg sulfadiazine/mg trimethoprim, respectively (Tribrissen, Di-Trim). The tablets are designated by the total weight of active ingredient in each tablet; thus Tribrissen 30 contains 25 mg sulfadiazine and 5 mg trimethoprim. The approved dose is 30 mg/kg orally or 26.4 mg/kg by subcutaneous injection daily for up to 14 days. The preferred dose for bacterial infections in dogs and cats is 30 mg/kg once or twice daily and may be indicated for severe coccidial infections. The manufacturer recommends that animals with marked hepatic parenchymal damage, blood dyscrasias, or previous sulfonamide sensitivity should not be given this product.8,10
Sulfamethoxazole with trimethoprim is a readily available product approved for use in people (Bactrim, Septra); it is not currently approved for use in animals. Because of its similarity to veterinary potentiated sulfonamides and because low-cost generics are available, it is widely used in veterinary medicine. There is some controversy regarding the appropriate dosing regimen for this human-labeled product in animals, but many clinicians gain acceptable clinical results using the same dose as sulfadiazine.
Sulfamethoxazole with trimethoprim is available in a fixed combination of 5:1 sulfamethoxazole to trimethoprim as tablets and pediatric suspension. The available single-strength tablets contain 400/80 mg and double-strength tablets contain 800/160 mg trimethoprim, respectively (Bactrim, Septra). The pediatric oral suspension contains 40 mg sulfamethoxazole and 8 mg trimethoprim per milliliter. The dose for bacterial infections and coccidiosis in dogs and cats is 30 mg/kg once daily for 10 days10 and may be indicated in severe coccidial infections.
Amprolium
At very high doses, amprolium may produce thiamine deficiency in the host. Thiamine deficiency can be treated by adding thiamine to the diet, although excessive thiamine supplementation may decrease the efficacy against the pathogen. In dogs adverse reactions are apparently rare and may consist of neurologic abnormalities, depression, anorexia, and diarrhea.10
Amprolium is approved for use in the drinking water or feed of poultry and cattle for the prevention and treatment of coccidia. Treatment for dogs and cats requires adapting the approved formulations to small animal use. The target dose for treatment of dogs is 100 to 200 mg/kg by mouth daily in food or water.10 Dogs may be treated by mixing 30 mL (2 Tbs) of 9.6% amprolium solution to 1 U.S. gallon (3.8 L) of drinking water and offering it as the sole source of drinking water.11 Alternatively, 1.25 g of 20% amprolium powder can be mixed with daily ration sufficient for four puppies.12 Amprolium should be provided in either the food or the water but not in both for a period of 7 days. It may be given as a treatment for coccidia or as a preventive measure for 7 days before puppies are shipped or to bitches just before whelping.
Cats may be treated at a dose of 60 to 100 mg/kg by mouth once daily for 7 days, which may be accomplished by direct oral administration.13 Placement of medication in food or water may be more unreliable for cats than for dogs owing to the finicky eating habits of many cats.
Furazolidone
Although the nitrofurans (nitrofurazone and furazolidone) have been reported in the literature as being effective in the treatment of coccidiosis and were once widely available for oral treatment of food animals, they have been systematically eliminated from the veterinary marketplace in the United States because of concerns regarding carcinogenicity. Furazolidone apparently inhibits numerous microbial enzyme systems, especially those related to carbohydrate metabolism, but the actual mechanism of action remains to be determined.14 Furazolidone is still available in a dosage form that is approved for human use (Furoxone). Potential toxicity includes gastrointestinal disturbance, peripheral neuritis, decreased spermatogenesis, and weight gain.15 Dogs and cats can be treated with 8 to 20 mg/kg orally, 1 to 2 times daily, for 5 days.10,13 The product is available in 2 formulations approved for use in people (Furoxone): 100-mg tablets and an oral liquid containing 3.34 mg/mL.
Toxoplasmosis
Biology
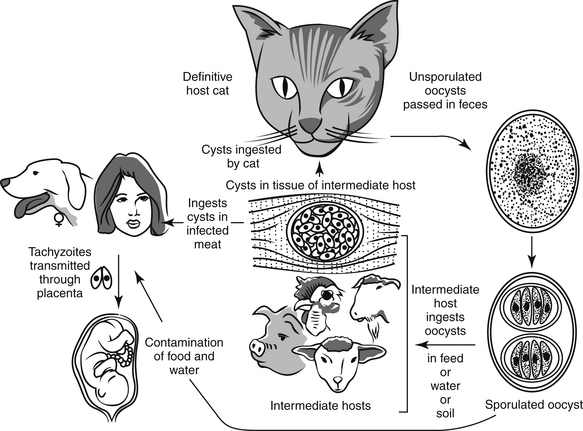
Toxoplasmosis is caused by Toxoplasma gondii, an obligate intracellular coccidian parasite. The parasite and the disease occur worldwide and have serious zoonotic impact. The domestic cat and other cats serve as the definitive hosts of this parasite. All other warm-blooded animals serve as intermediate hosts. In the United States, infection rates range from 0% to 100% in cats, 30% in dogs, and 30% to 60% in people. Although infection and seroconversion are common, clinical disease and diagnosis are rare.16
Enteroepithelial Life Cycle
The enteroepithelial life cycle of T. gondii in cats is similar to the life cycle of the common enteric coccidia (Figure 12-2). Toxoplasma oocysts are ingested from the environment; alternatively, tissue cysts may be ingested by carnivorism. Once ingested, bradyzooites are released that penetrate the epithelial cells and begin a cycle of asexual reproduction. The sexual stage of the cycle proceeds when the zooites differentiate into microgametes and macrogametes. The macrogametes are fertilized by the microgamete, and the resulting union produces an oocyst that is shed in the feces to begin the cycle again. It is believed that the enteroepithelial life cycle and the resulting oocysts occur only in cats; therefore only cats shed infective oocysts.
Extraintestinal Life Cycle
The extraintestinal life cycle occurs in all warm-blooded animals, including cats. This cycle begins when oocysts or infected tissues are ingested. The bradyzoites or sporozoites penetrate intestinal cells and undergo asexual reproduction and then break out of the gastrointestinal tract to infect virtually all other tissues, including the brain, striated muscle, and liver. After entering these extraintestinal tissues, they penetrate the cell and multiply until the cell is destroyed. The tachyzoites are released to infect other cells, and the cycle repeats. Eventually, the tachyzoites form tissue cysts that remain viable and infective for the life of the animal. These tissue cysts are infective to all warm-blooded animals and infect all animals who ingest the infected tissues. It is the ubiquitous tissue migration and replication across innumerable species that makes the pathogen so insidious and dangerous.
Clinical Signs
Clinical signs in cats are most severe in prenatally infected kittens. Such kittens may be stillborn or die before weaning. Clinical signs relate to pathology in the liver, lungs, and central nervous system. Adult cats typically demonstrate anorexia, lethargy, dyspnea, weight loss, icterus, vomiting, diarrhea, stiff gait, shifting leg lameness, or neurologic deficits. Ocular lesions may include uveitis in the anterior and posterior chambers, iritis, iridocyclitis, and detached retina. In severe cases respiratory or central nervous system involvement may cause death.17
Infected dogs may show clinical signs related to respiratory, neuromuscular, or gastrointestinal pathology. Generalized toxoplasmosis is characterized by fever, tonsillitis, dyspnea, diarrhea, and vomiting.17 More devastating clinical signs may be seen in dogs with neurologic or muscular involvement. Seizures, neurologic deficits, tremors, ataxia, paresis, or paralysis may be seen in these animals. Ocular lesions in dogs are infrequently reported.17
Clindamycin
Clindamycin is currently considered the drug of choice for treating toxoplasmosis. Structurally, clindamycin is a congener of lincomycin. Clindamycin is well absorbed (90%) after oral administration and is widely distributed in most tissues, except the central nervous system. It readily crosses the placenta and is extensively bound to plasma proteins. The drug is metabolized in the liver and excreted primarily in the urine and bile.18 Gastrointestinal upset is sometimes reported in animals receiving clindamycin. Severe, even fatal, pseudomembranous enterocolitis has been reported in people, caused by overgrowth of Clostridium difficile.
Treatment of systemic Toxoplasma infection in dogs can be accomplished with oral or intramuscular clindamycin at 10 to 20 mg/kg twice daily for 2 weeks.17,19 Cats can be treated for systemic infections with oral or parenteral clindamycin at 10 to 12.5 mg/kg twice daily for 2 to 4 weeks; this antimicrobial is also useful to control shedding of oocysts.20 The drug should be given with caution to cats with pulmonic toxoplasmosis; parenteral administration to experimentally infected cats resulted in several deaths.10
Sulfa Plus Pyrimethamine
Pyrimethamine can cause anorexia, malaise, vomiting, depression, and myelosuppression. Concomitant oral administration of folinic acid or brewer’s yeast may help alleviate some of these clinical signs. Because toxicity may develop rapidly in cats, they should have frequent hematologic monitoring. It is a teratogen in rats but is sometimes used by pregnant women.10
Dogs and cats are treated for systemic Toxoplasma infections at a dose of 30 mg/kg sulfa and 0.25 to 0.5 mg/kg pyrimethamine orally twice daily for 2 weeks. Cats may be treated to control shedding of oocysts at a dose of 100 mg/kg sulfa and 2 mg/kg pyrimethamine orally once daily for 1 to 2 weeks.17
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree