Chapter 21 Drug Therapy for Endocrinopathies
Drug therapy for the endocrine system is implemented to either replace a deficient hormone or prevent or reduce the formation or effects of an overabundant hormone. Hormones may also be administered to provocatively test for the presence of an endocrine disease. Understanding the proper uses of the drugs depends on an appreciation of the normal physiology of each endocrine system, including mechanisms of control and behavior of target tissues (Table 21-1).
Diseases of the Thyroid Gland
Synthesis of Thyroid Hormones
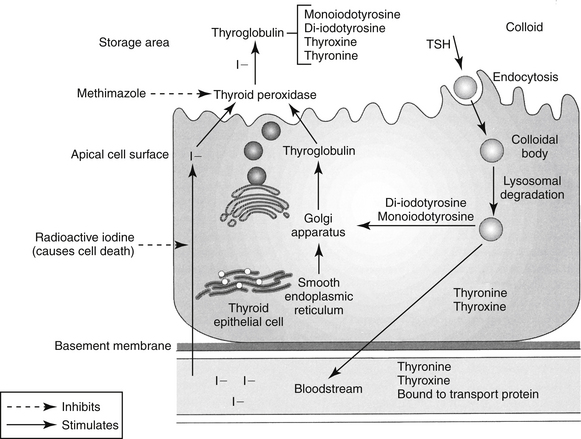
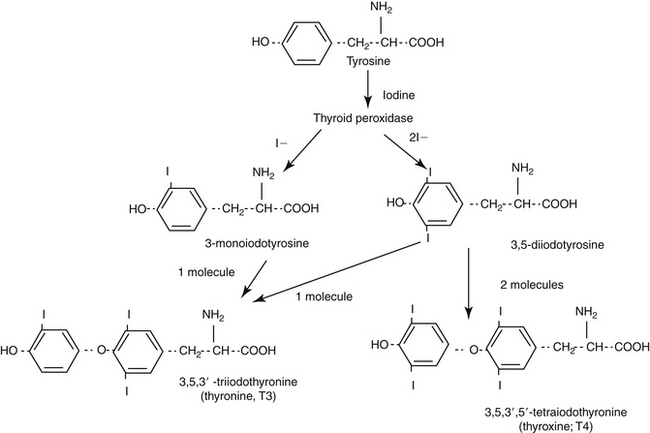
The protein thyroglobulin is synthesized by the endoplasmic reticulum and Golgi apparatus of thyroidal follicular cells and is released in vesicles into the colloid within follicular lumens. Iodine is accumulated by an active transport process in thyroidal follicular cells (Figure 21-1). Once in the follicular lumen, iodine is rapidly oxidized and combined with a tyrosine residue within thyroglobulin to form monoiodotyrosine. Monoiodinated tyrosines are then joined to form diiodinated tyrosines; further combinations yield triiodinated tyrosine (i.e., triiodothyronine [T3] and thyroxine [T4]), which contains four iodine molecules (Figure 21-2). The enzyme thyroid peroxidase mediates oxidation of iodine and formation of monoiodinated and diiodinated tyrosines as well T3 and T4. Four or more sites exist within the thyroglobulin molecule for the generation of thyroid hormones; generally, each molecule contains three or four molecules of T4 and zero to one of T3 (humans). The preformed hormones are released from the follicular colloid upon stimulation with TSH, with much greater quantities of T4 being released than T3. The hormones are transported bound to one of several transport proteins in the bloodstream and are delivered to target cells. At a target cell, both hormones are taken up. Intracellularly, T4 is deiodinated on its outer ring to produce T3, which is actually the active hormone that causes physiologic effects. In contrast, deiodination of the inner ring produces physiologically inactive reverse T3 (rT3). Thyroid hormones stimulate many metabolic processes, including activity of many enzymes; metabolism of vitamins and minerals; regulation of other hormones; and stimulation of calorigenesis, protein and enzyme synthesis, and carbohydrate and lipid metabolism. They also have marked cardiac inotropic and chronotropic effects, stimulate erythropoiesis, and affect virtually every body tissue.
Hypothyroidism
Pathophysiology
Myxedema coma is a rare endocrine emergency resulting from decompensation of severe chronic hypothyroidism. Myxedema refers to dermal accumulation of glycosaminoglycans, which bind water and cause an increased skin thickness and nonpitting edema, mostly in the face, jowls, and distal extremities.1 Myxedema coma is characterized by profound weakness, bradycardia, hypotension, hypoventilation, hypothermia without shivering, and altered mentation ranging from dullness and depression to stupor or coma. The actual mortality rate is not known but is thought to be high, primarily because myxedema coma is not widely recognized. The diagnosis should be made clinically, and therapy should be initiated without waiting for results of thyroid hormone concentrations.1 In addition to the biochemical abnormalities typical of hypothyroidism, additional findings may include hypoxia, hypercarbia, hyponatremia, and hypoglycemia. Serum thyroid hormone concentrations are typically very low or undetectable.
In humans the presence of concurrent disease and altered mentation are essential features for supporting a diagnosis of myxedema coma. In one study of seven hypothyroid dogs for which intravenous levothyroxine therapy was deemed necessary, five dogs had altered mentation. Of those five, all had concurrent disease and four had myxedema. Because of the low incidence of myxedema coma, it is not known if there is a breed predilection, but in a study by Pullen and Hess,2 three were Rottweilers, two were mixed breeds, one was a Cocker Spaniel, and one was a Shetland Sheepdog. Rottweilers were overrepresented. Treatment is with intravenous levothyroxine (discussed later).
Baseline and Provocative Testing of Thyroid Status
Diagnosis of hypothyroidism can be challenging. Hormones typically measured include T4, free T4, and TSH. If serum total T4 concentration is normal, it is highly unlikely that the dog is hypothyroid.3–6 Because nonthyroidal factors such as drugs, illness, and age affect T4, if the T4 concentration is below normal, the dog may or may not be hypothyroid,3–57 and further testing is required. Breed may also affect reference ranges.8,9,10
Serum TSH concentration can also be measured to help establish a diagnosis of canine hypothyroidism. In primary hypothyroidism TSH concentration should be elevated owing to lack of negative feedback of serum T4 on the pituitary (i.e., in normal dogs T4 feeds back and suppresses TSH secretion). Although 99% of canine hypothyroidism cases are believed to be due to primary thyroidal failure, only approximately 63% to 82% of hypothyroid dogs have elevated serum TSH concentrations.11–14 The reason for the discrepancy is unknown but is likely due to inadequacy of currently available assays. Of the hormones measured to diagnose hypothyroidism, however, TSH measurement is the most specific—that is, it has the smallest chance of a false-positive result.
Bovine TSH previously was used as the provocative agent for TSH stimulation testing but is no longer available. Thyrogen, recombinant human TSH (rhTSH), can be used instead. The optimal protocol for distinguishing normal and hypothyroid dogs, however, remains to be determined, although results of studies evaluating rhTSH in dogs are promising.15–17 Doses of 50 to 100 μg/dog given intravenously with samples taken before and at 4 or 6 hours after injection are likely appropriate. Euthyroid animals should respond to TSH stimulation with increased T4 concentration, whereas hypothyroid animals should not. Reconstituted rhTSH can be stored at 4°C for 4 weeks and at −20°C for 8 weeks without loss of biological activity.18
Measurement of serum T3 concentration is not helpful for diagnosing hypothyroidism. Most T3 is derived from intracellular metabolism or conversion by peripheral tissues of T4 to T3 and reverse T3 (rT3). Thus most body T3 is located within cells, and serum T3 concentration does not reflect total body levels. A previous argument for measuring serum T3 concentration was the belief that some dogs were “poor converters” and could produce T4 but not T3. The theory was apparently substantiated by finding normal serum T4 but nondetectable T3 concentrations in a small percentage of dogs. Such discrepant results are now known, however, to be an artifact caused by the presence of T3 autoantibodies. Insofar as poor conversion has never been proved and currently is not believed to exist, measurement of serum T3 concentration is not recommended.
Autoantibodies made against T4 or T3 can be measured and can be a marker of immune-mediated destruction of the thyroid gland. Autoantibodies typically cause spurious elevations in serum T3 or T4 concentrations, so their presence should be suspected if either hormone is measured in a patient suspected to be hypothyroid and the concentration of either or both hormones is reported to be greater than the reference range. However, the clinical and prognostic significance of autoantibodies is unknown. The presence of autoantibodies does not mean a patient is hypothyroid. If autoantibodies are suspected, serum fT4 concentration should be measured by equilibrium dialysis for the best assessment of function.19 If the serum fT4 concentration is within the reference range, thyroidal function is normal at that time but the patient should be reevaluated periodically (e.g., every 3 months) for development of hypothyroidism. If serum fT4 concentration is low, the dog is likely hypothyroid. One study followed 234 dogs with normal T4 and TSH levels and elevated antithyroglobulin antibodies (TGAA) for 1 year. Only 19% developed clinical signs of hypothyroidism or consistent laboratory values (or both). Another 57% remained TGAA positive without signs or laboratory evidence of hypothyroidism, 8% went from positive to borderline results, and 15% became TGAA negative.20
Calculation of the percentage of uptake of radioactive pertechnetate (99mTcO4–) has proved to be a useful tool aiding in the diagnosis of thyroidal illness in humans and cats and may also be valuable for dogs with primary hypothyroidism. Normal lobes of canine thyroid glands are uniformly intense, symmetric ovals, slightly smaller than the parotid salivary glands, and have smooth and regular margins. The parotid glands also concentrate 99mTcO4–, and the normal uptake ratio between these the thyroid and parotid glands is 1:1. In 14 dogs with histologically confirmed primary hypothyroidism, the percentage of uptake of 99mTcO4– distinguished hypothyroid dogs from dogs with nonthyroidal illness.21 The dogs with nonthyroidal illness had 99mTcO4– uptake ranging from 0.39% to 1.86%, similar to what has been described in healthy Beagles. The hypothyroid dogs had 99mTcO4– uptake ranging from 0.03 to 0.26%. In fact, of the tests examined in the study (T4, fT4, TSH, and TSH and TRH stimulation tests), scintigraphy was the only one that did not have overlap between the two groups of dogs. Limitations of the test include requirement for specialized equipment and the fact that reference ranges have not been defined in a large number of dogs from a variety of breeds. Additionally, increased 99mTcO4– uptake can occur with a diet high in iodine and has been reported in a hypothyroid dog with thyroiditis,22 so false-negative results are possible. The final complicating factor is that in humans with thyroiditis, the 99mTcO4– uptake pattern depends on disease stage. The role scintigraphy holds in aiding in the diagnosis of canine hypothyroidism remains to be defined.
Another area that has received recent attention as an ancillary test in the diagnosis of canine hypothyroidism is ultrasonography. In healthy dogs and dogs with nonthyroidal illness, the thyroid gland lobes are fusiform in the longitudinal plane and triangular in the transverse plane, have a smooth thyroid capsule, and homogenous echotexture, usually hyperechoic or isoechoic to the sternothyroid muscle.23,24 The ultrasonographic description of the thyroid gland lobes of hypothyroid dogs is more variable. However, they tend to be either round or ovoid on the transverse view. Echotexture may depend on whether the animal has thyroglobin autoantibodies. Thyroid lobes from antibody-positive hypothyroid dogs were homogenously hypoechoic to the sternothyroid muscle, whereas the lobes of those that were antibody negative were heterogeneous in echotexture.23 However, another study did not find a difference based on thyroglobin autoantibody status; thyroid lobes were reported to be hypoechoic to the sternothyroid muscle.24 Additionally the volume of the glands is smaller in hypothyroid dogs. A thyroid volume of less than 0.05 mL/kg was reported to be 81% sensitive, 96% specific, and 91% accurate for the diagnosis of primary hypothyroidism.23 Care should be taken with the use of this modality for diagnosing canine hypothyroidism because ultrasonography is highly user dependent and substantial interobserver variability exists when taking measurements.
Therapy of Hypothyroidism
Disposition of thyroid hormones
Thyroidal hormone disposition varies between preparations and species. Although thyroid hormones are bioavailable after oral administration, T4 absorption can be decreased by a number of factors such as the type of preparation and intraluminal contents of the ileum or colon, where most absorption occurs. In humans bioavailability ranges from 40% to 80%. Because of differences among formulations, some authors recommend initiating therapy with a brand-name product.25 However, the pharmacokinetics of T4 is idiosyncratic; some dogs achieve higher postpill concentrations when treated with a generic brand. No evidence exists that a single brand is consistently better than another. Therapy can probably be started with any brand, but if a dog does not respond, another brand should be administered to see whether better results and higher postpill levels can be achieved; if the first brand tried is generic, the second should not be. In contrast to T4, T3 is well absorbed (95% in humans) from the gastrointestinal tract.
In dogs the half-life of T3 and T4 is 5 to 6 hours and 12 to 15 hours, respectively. However, the half-life of T4 may be dose dependent.26 The short half-life makes avoidance of fluctuations in serum concentrations during a 12- to 24-hour dosing interval difficult. However, the status of intracellular T3 concentration is not well known; the impact of fluctuating T4 may be minimized by physiologic conversion. Steady-state concentrations will occur at five drug half-lives (i.e., 75 hours for T4 and 30 hours for T3 in dogs). On the other hand, clinical response varies depending on the complication of hypothyroidism present. The first evaluation after starting supplementation should occur after about 4 weeks of therapy in order to judge blood levels as well as therapeutic response. Certain abnormalities (e.g., dermatological) can take up to 3 months to resolve once therapeutic blood levels have been achieved.
Preparations
Thyroid hormones can be supplemented as crude extracts of animal origin or as synthetic preparations. The biological activities of animal-origin products, such as desiccated thyroid and thyroglobulin, vary, however, and therapeutic failure with these products is not uncommon. Thus synthetic products are recommended.
Several synthetic thyroid hormone products are available as T4 or T3 individually or in combination. Sodium levothyroxine (T4) is the drug of choice for most patients. If T4 therapy has failed to achieve a response in a dog with confirmed hypothyroidism, T3 can be administered, but failure of T4 therapy is very rare. If no apparent response to T4 is seen or inadequate blood levels are achieved with appropriate dosing, another brand of T4 should be used first before prescribing T3. The diagnosis of hypothyroidism should also be reevaluated if resolution of clinical signs does not occur with documentation of adequate blood concentrations and a sufficient duration of therapy. Combination products generally contain T4 and T3 at a ratio of 4:1, the proportion of thyroid hormones secreted in normal humans. Use of combination products is not recommended. The administration frequency of T4 and T3 should differ, orally absorbed T4 is converted as needed to T3 by target cells so therapy with T4 alone usually achieves adequate T3 concentrations, and use of combinations may result in serum T3 concentrations that produce thyrotoxicosis.1
Pharmacokinetics of oral liquid levothyroxine suggest that once-daily dosing should be adequate,27 and administration of liquid levothyroxine (Leventa Intervet/Schering-Plough) once daily in 35 hypothyroid dogs was recently evaluated.28 Dogs were started at a dose of 20 μg/kg once daily and monitored every 4 weeks (adjustment phase). At the reevaluation visit, serum T4 and TSH concentrations were obtained 4 to 6 hours after the pill was administered, and dose adjustments were made if the T4 value did not fall within the target therapeutic range. Once clinical signs resolved and T4 concentrations were within the target range, the adjustment phase ended and the maintenance phase began. To assess long-term efficacy, dogs were then reevaluated at 9 and 22 weeks, with the same parameters measured at each visit and dosages adjusted as needed.
Intravenous levothyroxine is used in the treatment of dogs with myxedema coma. In 7 dogs given intravenous levothyroxine, the median dose was 7 μg/kg. Three of the seven dogs were given intravenous levothyroxine once. To resolve the neurologic issues, the remaining dogs received a total of 5, 6, 10, or 13 doses each. The injections were given every 12 hours in three of four dogs and every 8 hours in the remaining dog. The dogs were started on oral levothyroxine within 24 hours of the last intravenous dose.2
Response to Therapy
Therapeutic Drug Monitoring
For monitoring a sample should be drawn 4 to 6 hours after the pill is administered. At that time, serum T4 concentration should be in or slightly above the upper half of the reference range. TSH concentration can also be measured in the same sample and can be helpful. Increased values are associated with inadequate therapy, but TSH concentrations within the reference range are not interpretable; dogs with both adequate and inadequate control can have TSH concentrations within the reference range.29 Measurement of fT4 probably is unnecessary and does not add any more information, except in patients who have T4 autoantibodies; in such patients T4 concentration as measured by radioimmunoassay is falsely elevated. Whether measurement of prepill hormone concentrations is helpful remains controversial. If T3 is the sole supplement, peak concentrations can be collected 3 hours after administration of the pill if an 8-hour dosing interval is being used. With subsequent retesting, samples should be collected at the same time as previously, so comparisons between tests across time in the same patient are more valid.
Interpretation of thyroid hormone concentrations must be made in the context of clinical signs. Animals should be supplemented for 1 to 3 months before clinical efficacy can be judged. Although no clinical signs of thyrotoxicosis may be present, whether increased serum T4 concentrations without clinical signs of hyperthyroidism are detrimental has never been studied. In humans elevated postpill T4 concentrations are strictly avoided. Low serum T4 concentrations should also be interpreted with response to therapy. The effects of concurrent drug therapy or other diseases that might influence the metabolism of thyroid hormones must be considered. Drug dose can be changed to achieve the therapeutic range. However, the pharmacokinetics of T4 are not linear—that is, to achieve a 15% change in serum T4 level, a 25% change in dose, not a 15% change, may be required.30
Hyperthyroidism
Baseline and Provocative Testing
Baseline serum T4 concentration can be measured to diagnose hyperthyroidism and is sufficient in the majority of cases as it is elevated in approximately 91% of hyperthyroid cats.33 (It is important to remember, however, that rare normal cats may have an elevated T4.) The other 9% of cats represent those with early or mild hyperthyroidism where serum T4 concentration can fluctuate in and out of the normal range34 or cats with concomitant nonthyroidal illness. Nonthyroidal illness can suppress serum T4 concentration,35 so the serum T4 concentration in hyperthyroid cats can be within the normal range.36,37 As a result, resting serum T4 concentrations obtained from cats must be critically evaluated and the possibility of further testing considered.
If the serum T4 concentration of a hyperthyroid cat is within the reference range, it will typically be in the upper half of the range. If a serum T4 concentration is in the lower half of the reference range, it is highly unlikely (but not impossible) that the cat is hyperthyroid, and another diagnosis should be considered. In sick, older cats with normal thyroidal function, serum T4 concentration is usually low,35 so the finding of a T4 even in the upper half of the reference range in such a cat may indicate hyperthyroidism. If the T4 concentration is in the upper half of the reference range but there hyperthyroidism is still suspected, further diagnostics should be pursued. Options for additional tests include the following: (1) measurement of serum fT4 concentration by equilibrium dialysis; (2) repeat measurement of total T4 concentration (Because serum T4 concentration may fluctuate in and out of the normal range, on a second test the sample may be drawn by chance while the T4 concentration is above normal and diagnostic. Unfortunately, there is no way to predict when this will occur.); (3) performance of a T3 suppression test or TRH stimulation test. These latter two tests are valid, good tests, although they are more labor intensive and often not necessary.
As in dogs, serum fT4 concentration in cats is less affected by nonthyroidal factors than is total T4 concentration and is a more accurate reflection of thyroid function. For example, fT4 concentration is elevated in 94% of mildly hyperthyroid cats, whereas total T4 concentration is elevated in only 61%.33 However, fT4 concentration may also be elevated in 6% to 12% of sick, euthyroid cats.33,38 To help discriminate between hyperthyroid and sick euthyroid cats, a total T4 should be measured along with fT4 concentration. Sick euthyroid cats with an elevated fT4 typically have T4 concentrations that are within the lower half of or below the reference range. Thus if the T4 concentration is in the upper half of the normal range or above and the fT4 concentration is elevated, this is consistent with a diagnosis of hyperthyroidism. If the T4 concentration is in the lower half of the normal range or below and the fT4 concentration is elevated, the cat is very unlikely to be hyperthyroid, and another diagnosis should be sought.
Measurement of serum T3 is not very helpful for diagnosis and is not recommended. Overall, only 67% of hyperthyroid cats have an elevated T3 concentration.33 In cats with mild hyperthyroidism, only 21% actually have an elevated serum T3 concentration.33
Performance of pertechnetate scans to diagnose hyperthyroidism is quite useful overall. Uptake of radioactive pertechnetate can confirm hyperthyroidism as well as delineate functional thyroid tissue, establish the extent of thyroid involvement, and possibly detect metastasis (some metastases, but not all, will concentrate radioiodine). For cats in which biochemical evidence of hyperthyroidism is clear, scans will clearly identify functioning tissue. However, false-positive results may occur;39 in other words, nonhyperthyroid cats can have a scan suggesting the presence of hyperthyroidism, and timing of a scan after methimazole therapy may affect results.40
Relationship of Treatment for Feline Hyperthyroidism to Renal Disease
Treatment of hyperthyroidism can lead to decreases in GFR and unmask chronic renal disease,41–45 and no therapy appears to be safer than another. However, how best to assess cats before definitive therapy (i.e., radioactive iodine [131I therapy] or surgery) is unknown. Although one study determined that a GFR value of 2.25 mL/kg/min might represent a cutoff for deciding whether renal failure is a possibility,43 measurement of GFR is not easily obtained and GFR measurements vary depending on the technique used,46 with each laboratory needing to determine its own guidelines. Furthermore, in two studies, hyperthyroid cats with GFR greater than 2.25 mg/kg/min were azotemic 30 days after treatment;42,44 whether this simply reflects a difference in methodology is unclear. Unfortunately, no readily available clinical indicators, including urine specific gravity, exist.47,48 Cats with pretreatment urine specific gravity greater than 1.035 may develop azotemia with treatment.48
Whether all cats that are to undergo 131I treatment or thyroidectomy need to have their kidney function evaluated with a methimazole trial remains to be determined. We prefer performing a trial on all cats before definitive therapy is undertaken. Certainly, if there is any question about the adequacy of renal function, trial therapy with methimazole is warranted. If renal failure becomes clinically apparent during the trial, methimazole administration should be stopped and therapy for renal failure instituted. Once the cat is stable again, the hyperthyroidism should be controlled as best as possible for life with methimazole and therapy for renal failure continued. Alternatively, if a trial is not performed and renal failure becomes overt because of definitive correction of hyperthyroidism, exogenous thyroid hormone can be supplemented in an attempt to support the kidneys, but the efficacy of such therapy is unknown and may be questionable.49 A balance must then be struck between creating iatrogenic hyperthyroidism and maintaining renal function.
Drugs Used To Control Hyperthyroidism
Hyperthyroidism may be medically controlled with methimazole and ipodate. Both can be used as either the sole drug to manage hyperthyroidism or in preparation for surgery or radioiodine administration. Propylthiouracil administration is not recommended because of associated possible severe adverse effects. In general, methimazole blocks synthesis of thyroid hormones and, specifically, thyroid peroxidase activity necessary for coupling of tyrosine residues by acting as a preferential substrate for the enzyme. As a result, T3 and T4 are not secreted. Carbimazole is a methimazole prodrug currently used in Europe but not available in the United States. It appears to be equal in efficacy but safer than methimazole. Controlled-release carbimazole tablets are now available in Europe. Based on pharmacokinetics in normal cats the controlled-release formulation may be appropriate for once-daily dosing;50 however, dosing has not been evaluated in hyperthyroid cats. Ipodate is a cholecystographic agent that acts primarily by inhibiting conversion of T3 to T4 but also has some direct inhibitory effects on thyroid hormone secretion.
Overall, methimazole is highly effective at reversing thyrotoxicosis and maintaining euthyroidism. In 262 spontaneously hyperthyroid cats, methimazole treatment lowered the serum T4 in more than 99%.51 A very small percentage of cats may be truly methimazole resistant. In the 262 cats, clinical side effects occurred, unrelated to the dose of methimazole used, in 18%, including anorexia (11%), vomiting (11%), lethargy (9%), excoriation of the face and neck (2%), bleeding (2%), and icterus (2%). Anorexia, vomiting, and lethargy typically happened during the first month of therapy and resolved despite continued drug administration. However, in eight cats, gastrointestinal side effects persisted and required cessation of therapy. Treatment with methimazole was also permanently stopped in cats that developed liver failure (e.g., vomiting, anorexia, and icterus), excoriated faces or necks, or a bleeding tendency.51 Myasthenia gravis has been reported after treatment with methimazole in four cats. In two cats, prednisone was used to control the myasthenia.52 Lymphadenomegaly, which resolved with discontinuation of methimazole administration, was reported in a single cat.53
On hematologic screening, eosinophilia, lymphocytosis, leukopenia, thrombocytopenia, and agranulocytosis may be noted. The milder adverse effects—eosinophilia, lymphocytosis, and leukopenia—are usually noted within 1 to 2 months of initiation of treatment and are transient despite continued therapy. The more serious complications (e.g., thrombocytopenia, agranulocytosis) occur in a minority of cats (≤3%) within the first 3 months of therapy and necessitate permanent discontinuation of methimazole administration.51 The mechanism of hematologic disorders induced by methimazole is not understood. Interestingly, bleeding occurred in one cat without a decrease in platelet number, so thrombocytopenia is not the only mechanism that can cause a bleeding tendency. In human patients receiving propylthiouracil, vitamin K therapy reduced bleeding caused by hypoprothrombinemia; however, the benefits of vitamin K therapy have not been studied in cats receiving thyroid peroxidase inhibitors.54 Immunologic effects, including induction of positive antinuclear antibodies (ANAs), can occur. The risk of developing a positive ANA result appears to increase with length of therapy and dose. However, clinical signs of a lupuslike syndrome (e.g., dermatitis, polyarthritis, glomerulonephritis, thrombocytopenia, fever) or hemolysis do not occur.51
A starting dose of 10 to 15 mg/day divided into two or three daily doses depending on the severity of the hyperthyroidism has been recommended. The goal for cats on methimazole is to have a serum T4 concentration in the lower half of the reference range. Postpill timing does not matter. Although some cats require methimazole only once daily for adequate control, methimazole generally is more effective twice daily.55 For the first 3 months, the period during which most adverse effects develop, cats receiving methimazole should be evaluated every 2 to 3 weeks with a complete physical examination, determination of serum T4 concentration, complete blood count (CBC), and measurement of liver enzymes and bilirubin. Renal parameters should also be monitored to assess kidney function. Although cats with a subnormal serum T4 concentration typically are not clinically hypothyroid, development of a positive ANA titer may be related to dose. Thus the minimal dose necessary to maintain serum T4 concentration in the lower half of the reference range, and not below, should be used. If serum T4 concentration remains high and poor compliance or difficulty in giving the medication has been ruled out as the cause of persistent hyperthyroidism, the methimazole dose should be increased in 2.5- to 5-mg increments to a maximum of 20 mg/day. If hepatopathy, facial excoriation, a bleeding tendency, or serious hematologic consequences occur, the medication should be halted permanently and alternative therapy used. After the first 3 months, serum T4 concentration should be determined every 3 to 6 months to evaluate adequacy of therapy. Because blood dyscrasias are unlikely but not impossible after 3 months of therapy, a CBC need be performed only if clinical signs suggest agranulocytosis, hemolysis, or thrombocytopenia.51
To prevent development of adverse effects, other authors have recommended an initial dose of 2.5 mg twice daily for 2 weeks.56 If after this period an owner observes no untoward side effects, the physical examination reveals no new problems, and a CBC (including platelets) is within normal limits, the dosage should be increased to 2.5 mg thrice daily for an additional 2 weeks. A similar recheck should then be completed, including measurement of a serum T4 concentration. If serum T4 concentration is within or near the normal reference range, the dose may be maintained for 2 to 6 weeks to determine the need for any further dosage adjustments. The dosage should be increased by 2.5 mg/day increments to a maximum of 20 mg/day (assuming correct methimazole administration) or until the hyperthyroidism is controlled.56 Monitoring for adverse effects should be done as previously described.
Because of the relationship between hyperthyroidism and renal disease (as previously discussed), a third protocol has been advocated if abnormal renal parameters are present. Methimazole should be administered at a dose of 2.5 mg twice daily for 2 weeks, then 2.5 mg thrice daily for 2 weeks, then 5 mg twice daily for 2 weeks, and finally 5 mg thrice daily as needed. The serum T4 concentration, BUN, creatinine, phosphate, and a CBC should be evaluated at the end of each 2-week period. The dose escalation should stop once serum T4 concentration has normalized. If the serum T4 can be decreased to within the reference range and the renal parameters remain stable or improve, antithyroid medications may be continued or a permanent therapy may be considered. If clinical signs of renal disease worsen with therapy, treatment of the hyperthyroidism should be reevaluated. Some cats may be healthier without treatment.56 Alternatively, the dose of methimazole can be titrated to achieve the best control possible of the hyperthyroidism while maintaining adequate renal function.
Methimazole can be given transdermally. Although methimazole in pleuronic lecithin organogel (PLO) is absorbed poorly in healthy cats after a single dose,57 it is likely that chronic dosing leads to improved absorption and resolution of hyperthyroidism as transdermal methimazole can be used to treat hyperthyroidism.58–60 The transdermal route may take longer, however, to bring about remission. In a randomized, prospective study of hyperthyroid cats, owners dosed their cats with methimazole orally (tablets) or transdermally (in PLO; 50 mg/mL) at 2.5 mg every 12 hours.59 Of cats treated transdermally 56% were euthyroid at 2 weeks, which was significantly fewer cats than the control rate in response to oral administration (88%); by 4 weeks the difference was no longer statistically significant (67% control with transdermal methimazole versus 82% for oral), but the lack of difference may have been due to a small number of cats remaining in the study at 4 weeks. Whether transdermal administration for a longer period of time would have controlled the hyperthyroidism in more cats was not evaluated. An advantage of transdermal methimazole is a significantly decreased rate of gastrointestinal adverse effects. However, the incidence of hepatopathy, facial excoriation, and blood dyscrasias is similar for both the transdermal and oral routes. Some cats develop erythema at the transdermal dosing site, but it is typically not severe enough to require drug discontinuation.59
Methimazole administration does not affect tumor size. Clinical signs will recur with discontinuation of the drug. Methimazole can be used before surgery to decrease serum T4 concentrations to within the reference range to stabilize the patient. Discontinuation of methimazole 2 weeks before radionuclide scanning or therapy has been recommended. Because radionuclide uptake is increased in normal tissue for 9 days after discontinuation of methimazole therapy,40 treatment within that window may increase the risk of iatrogenic hypothyroidism with radioiodine administration.
In humans, use of cholecystographic agents for treatment of hyperthyroidism has been studied, and the radiopaque organic iodine agent ipodate has shown some success. Experience with ipodate in veterinary medicine has been limited. A single study of 12 spontaneously hyperthyroid cats was performed using calcium ipodate granules reformulated into 50-mg capsules.61 Initial dosage was 50 mg/cat, administered orally twice daily. The dosage was increased to 150 mg (100 mg in the morning, 50 mg at night) and then 200 mg (100 mg orally twice daily) at 2-week intervals if serum T3 did not normalize or if other abnormalities attributable to hyperthyroidism failed to resolve satisfactorily. (Because the main mechanism of action of ipodate is to inhibit conversion of T4 to T3, monitoring of serum T3 concentrations is necessary to judge efficacy.) Only eight cats responded during the 14-week study period; seven of these were treated at a dosage of 100 mg/day, and one required 150 mg/day. Interestingly, serum T3 concentration decreased in the responders and declined into the normal range in two nonresponders as well. No adverse clinical signs or hematologic abnormalities attributable to ipodate treatment were noted.61 The reason for poor response in four cats was not apparent. Lack of efficacy of ipodate has been noted in human patients, especially those with Graves’ disease and severe hyperthyroidism. The effect of ipodate may be transient in some cats, given that two cats clearly had relapsed at the end of the 14 weeks.
Drugs Used To Cure Hyperthyroidism
Compared with medical therapy that simply controls hyperthyroidism, 131I therapy provides a cure. The goal of 131I therapy is to restore euthyroidism with a single dose of radiation without producing hypothyroidism. Surgery can also cure the disease, but 131I is the definitive treatment of choice in cats with ectopic thyroid tumors that are not surgically accessible (e.g., intrathoracic). In addition, 131I may be the best way to treat metastatic carcinoma. Radioactive iodine, like stable iodine, is actively taken up by and stored in thyroidal tissue. The emitted β particles cannot travel far, thus limiting damage to adjacent normal tissues. Because normal tissue has atrophied and is quiescent, 131I will be concentrated within a thyroid tumor(s), and normal tissue is relatively spared from destruction by the radioactive particles.
The 131I dose can be administered orally, subcutaneously, or intravenously but is usually given subcutaneously to avoid the stress of intravenous catheterization or injection and the possibility of vomition of radioactive material. The major disadvantages of radioactive iodine therapy include accessibility (a limited number of facilities are licensed to use radiopharmaceuticals, but this is changing), cost, and the possible extent of hospital stay while radioactivity decreases in the patient (potentially up to 2 weeks, depending on local radiation safety regulations). After discharge, cats will continue to excrete a small amount of radiation for 2 to 4 weeks, and close contact with the cat should be minimized during this time.
TSH increases 131I uptake of the thyroid gland in humans, and rhTSH is routinely administered before 131I treatment to lessen the 131I dose and minimize irradiation of nonthyroidal tissues. Whether rhTSH would increase the uptake of 131I in thyroid glands of hyperthyroid cats was recently examined.62 Five hyperthyroid cats were given 25 μg rhTSH intravenously 1 hour before 123I administration; 123I can be used to visualize hyperfunctional tissue and will be handled by the body like 131I but will not cure hyperthyroidism. The same cats were given another 131I injection 8 days later without first receiving rhTSH. The rhTSH increased radioactive iodine uptake by the thyroid gland by 7.33%, which was statistically significant. Thus hyperthyroid cats undergoing 131I therapy may require a lower radioactive iodine dose if given rhTSH before treatment, but further studies are needed to optimize the rhTSH dose and the interval between its administration and the 131I injection.
Administration of 131I by any route appears to be relatively safe. Pain or discomfort in the area of the thyroid gland presumably reflects radioactive thyroiditis and should resolve within several days of therapy. A transient voice change has been noted in one cat63 and transient dysphagia in eight.64 Although many cats develop a subnormal serum T4 concentration following 131I administration, a low serum T4 concentration in itself does not mean hypothyroidism is present. Because nonthyroidal diseases can suppress serum T4 concentration, nonthyroidal disease should always be excluded before a diagnosis of hypothyroidism is made. Supplementation is required only if clinical signs of hypothyroidism develop. Although 11% of cats have a low serum T4 concentration after 131I therapy, approximately only 2% require l-thyroxine supplementation.64
Clinical signs of euthyroidism generally occur within 1 to 3 weeks of 131I administration; the first sign generally is normalization of appetite and weight gain. Therapeutic failure rate is approximately 2%. If T4 is still elevated 3 to 6 months after 131I administration, the hyperthyroid state is unlikely to resolve without further treatment. Most of these cats will respond to a second dose of 131I.63–67 In one study 2.5% of cats had a relapse of hyperthyroidism 1.1 to 6.5 years after initial radioiodine treatment.64
Percutaneous ultrasound-guided intrathyroidal injection of ethanol or percutaneous radiofrequency ablation of thyroidal tissue has been tried as a means of targeting and destroying thyroid tumors. After treatment of unilateral disease with percutaneous ethanol injection (PEI), resolution was obtained lasting at least 12 months.68 However, only a small number of cats were studied. Bilateral PEI for bilateral disease led to mortality in one cat, likely because of laryngeal paralysis. Treating bilateral disease with staged injections has not shown long-term success, with the longest remission obtained being 27 weeks.69 Adverse effects include mild gagging, voice change, Horner’s syndrome, and laryngeal paralysis; are usually transient; and resolve in 8 weeks or less.68,69
Radiofrequency ablation was used to treat four cats with unilateral thyroid disease and five cats with bilateral disease.70 The nine cats were administered 14 treatments. Of the cats with unilateral disease, three became clinically and biochemically euthyroid for 1, 6, and 18 months; the fourth cat became clinically euthyroid for 6 weeks, although the serum T4 concentration remained slightly above the reference range. Interestingly, all cats with bilateral disease had treatments on one side and remission was obtained after 1 to 3 treatments for 1 to 6 months. Repeat therapy was used in some cats to induce a second or third remission when clinical signs returned. Transient Horner’s disease was the sole clinical complication and was seen after 3 of the 14 treatments. Clinically inapparent laryngeal paralysis was noted in one cat during laryngeal examination.70
Drugs Used To Control Clinical Signs of Hyperthyroidism
Thyroid hormones may increase the number or sensitivity of β-receptors in the myocardium.56 Tachycardia, myocardial hypertrophy, heart failure, and cardiac arrhythmias have been associated with thyrotoxicosis in hyperthyroid cats. Beta-adrenergic blockers (e.g., propranolol) have no effect on thyroid hormone concentration but decrease the neuromuscular and cardiovascular effects of hyperthyroidism, such as hyperexcitability, hypertension, and cardiac hypertrophy. These agents can be used in combination with an antithyroid drug such as methimazole or alone if a patient cannot tolerate antithyroid medications, and they may be helpful in preparing a patient for thyroidectomy or radioactive iodine by making the cat a better candidate for surgery or hospitalization. Nonselective β blockade by propranolol can reduce the hyperdynamic effects of thyroid hormones on the myocardium. In addition, propranolol inhibits conversion of T4 to T3 by peripheral tissues in hyperthyroid humans. Because propranolol does not directly affect the thyroid gland, however, patients are not returned to a euthyroid state. Propanolol dosing in cats suffering from cardiac disorders associated with hyperthyroidism should begin at 2.5 mg orally every 12 hours and be increased to 7.5 mg every 8 hours as necessary to control heart rate. If propranolol is being used to prepare a patient for surgery, therapy should continue for 14 days preoperatively. Care should be taken in patients with congestive heart failure because the negative chronotropic effects of propranolol may decrease myocardial reserve. In addition, as a nonselective β blocker, propranolol can cause bronchospasms, which may be lethal in cats with respiratory distress or may exacerbate feline asthma. Atenolol, a selective β1blocker, might be used instead of propranolol (2 mg/kg or 6.25 mg/cat once daily).
Administration of large doses of iodide (e.g., sodium or potassium iodide) for a short time (1 to 2 weeks) will cause transient hypothyroidism in normal animals. Organification of thyroid hormones is prevented and hormone secretion is reduced. The clinical effects of high-dose iodine therapy will occur in 7 to 14 days in humans; however, refractoriness to these effects will develop in several weeks to months. In hyperthyroid cats, iodine (50-100 mg orally, once daily) has been used to prevent an acute thyroid crisis (i.e., a thyroid storm) in patients undergoing thyroidectomy. One to two drops of a saturated solution of potassium iodide can be administered in gelatin capsules beginning 10 days before surgery.71
Diseases of the Parathyroid Glands
Normal Calcium Homeostasis
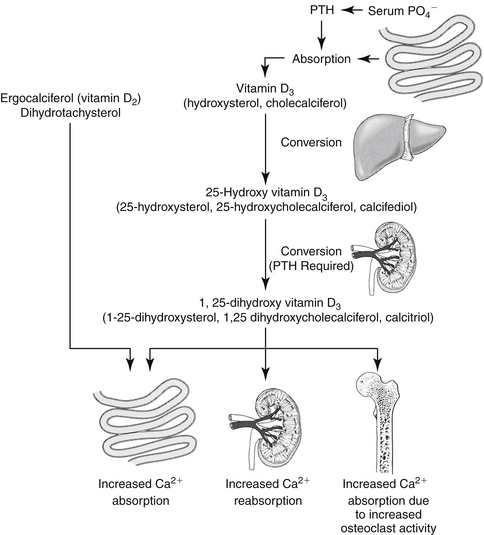
Calcium balance is maintained by the integrated influences of parathyroid hormone (PTH) on calcium and phosphorus reabsorption in bone and distal renal tubular cells and by the intestinal absorption of calcium as mediated by vitamin D (Figure 21-3). Of the total calcium present in serum, approximately 40% is protein bound, 10% is bound to other factors such as citrate or phosphate, and 50% is ionized. Serum ionized calcium concentration, which is the biologically active portion, normally fluctuates less than 0.1 mg/dL. Secretion of PTH is exquisitely sensitive to changes in ionized calcium concentration. Decreases in serum calcium concentration stimulate PTH secretion, which in turn causes increased calcium resorption from urine (distal renal tubule), increased mobilization of calcium and phosphorus from bone, and increased vitamin D synthesis. Parathyroid hormone mediates the activation of vitamin D (see Figure 21-3).
The main function of vitamin D is to increase gastrointestinal calcium and phosphorus absorption. In humans vitamin D (cholecalciferol) can be ingested or made by irradiation of cutaneous 7-dehydrocholesterol; the serum half-life of vitamin D is 19 to 25 hours, although the vitamin is stored in fat depots. In dogs vitamin D must be ingested; what occurs in cats is unknown. Cholecalciferol is converted to 25-hydroxycholecalciferol (25-OHD, calcidiol) in the liver by hepatic microsomal enzymes (see Figure 21-3). Once in circulation, 25-OHD is bound to a binding globulin. In humans 25-OHD has an elimination half-life of about 19 days. Calcidiol is further hydroxylated to the most potent form, 1,25-dihydroxycholecalciferol (calcitriol), in the renal proximal tubules. The enzyme responsible for hydroxylation of 25-OHD is inhibited by calcitriol in a negative feedback manner and by hyperphosphatemia. On the other hand, conversion of 25-OHD to calcitriol requires PTH; thus without PTH, little to no calcitriol is made and there is practically no functioning vitamin D in the body. Renal and, to some degree, bone activities mediate the acute response to calcium homeostasis. Intestinal calcium reabsorption may take several days to occur, in part because of the time necessary for vitamin D synthesis or activation. Calcitriol is further hydroxylated to 1,24,25-(OH) 3-D3 and subsequent metabolites that have variable activity.
Because ionized Ca is available to cells and is thus the biologically active form, it is critically important in the diagnosis of calcium disturbances. If ionized calcium is normal, even if total calcium is not, no further diagnostics are warranted. The correction formulas previously advocated and used to correct calcium concentration for serum albumin or protein concentration are no longer recommended.72 To know a patient’s calcium status, the clinician must measure ionized calcium concentration.
Hypoparathyroidism
Pathophysiology and Diagnosis of Primary Hypoparathyroidism
Primary hypoparathyroidism in dogs can reflect destruction of the parathyroid glands by disease (e.g., lymphocytic parathyroiditis) or trauma, including surgical removal. The most common cause in cats is injury or removal of the parathyroid glands during thyroidectomy. Hypomagnesemia can be a cause or effect of hypoparathyroidism in dogs and cats. Cessation of PTH secretion results in the loss of calcium mobilization from bone, of calcium retention by the kidneys, and of calcium absorption from the intestines. The primary clinical manifestation of hypoparathyroidism reflects decreased serum calcium concentration.
Hypoparathyroid patients may be hyperphosphatemic as a result of decreased renal phosphorus excretion. Measurement of serum PTH concentration is required to establish a diagnosis of hypoparathyroidism. Serum PTH concentrations below the reference range in hypocalcemic dogs and cats confirm the diagnosis, assuming the assay used is reliable and validated. Serum PTH concentrations in the low end of the reference range are not appropriate in hypocalcemic individuals, and a diagnosis of hypoparathyroidism can also be made.73
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree