Drug Therapy in Llamas and Alpacas
Making the decision to use pharmacotherapeutics and then selecting the drug and how to use it are fundamental competencies for the veterinarian. Acquiring these skills in veterinary practice with llamas and alpacas are challenging because no drugs have been approved in the United States for these species.1,2 Therefore, all drug use information must come from published data or extrapolation from other species. This chapter provides a framework for the veterinarian to evaluate these data. The framework includes (1) features that might affect drug selection and extrapolation of drug-related data from other species, including physiology, anatomy, clinical setting, and caregiver characteristics; (2) principles for reviewing published evidence for drug use decisions, for extrapolating from other species, and for selecting antimicrobial drug therapy. This chapter also provides already published data on which decisions with regard to drug selection are based. The collation of published data provides support for successful drug therapy in llamas and alpacas, with data categorized according to strength of evidence. Box 34-1 contains definitions for the abbreviations used throughout this chapter.
Framework for Evaluating Evidence about Drugs and Drug Selection
Features Affecting Drug Selection and Use
Anatomy and Physiology
Anatomic and physiologic factors that have the potential to affect drug selection and use include any factor that could affect pharmacokinetics, including absorption rate and extent, composition of tissues that might affect distribution of drug, metabolic processes such as hepatic metabolism, and organ and other factors that might affect elimination. One effect of the physiologic factors is the selection of route of administration of drug. Llamas and alpacas are functionally similar to ruminants in that they have chambers in the digestive tract that allow for anaerobic fermentation and utilization of cellulose-based plant materials for nutrition.3 This does not translate, however, into similar absorption rates of drugs administered orally, which means that regimens for oral dosage form drugs cannot be assumed to be the same from ruminant dosages. For example, at a similar dose rate (llamas were dosed with an average 59 milligrams per kilogram [mg/kg], orally [PO] and cattle with 55 mg/kg PO), maximum concentration (Cmax) was higher in cattle (approximately 70 microgram per milliliter [mcg/mL] based on visual interpretation of graphic data) than llamas (mean 22 mcg/mL).4,5 In the same studies, absorption half-life was approximately 4 hours in cattle compared to 11 hours in llamas. These differences are likely related to the physiologic characteristics of the llama stomach as well as other physiologic differences that result in differences in the distribution of drug throughout tissues (volume of distribution at steady state [VDss] for cattle was reported as 0.31 liter per kilogram [L/kg] compared with 0.44 L/kg reported in llamas). Another example is oral dosing of phenylbutazone: In llamas, the mean absorption time was 9.8 hours (although variability was high), whereas in cattle, half-life of absorption was reported as approximately 4 hours.6–8 Elimination of drugs occurs most commonly via hepatic metabolism, elimination via the kidney (filtration or tubular excretion), or both, so any differences between these species and others have the potential to affect pharmacokinetic parameters. The glomerular filtration rate (GFR) of two llamas was evaluated and found to be approximately 1 milliliter per kilogram per minute (mL/kg/min), similar to the reported values in dromedary camels, a species commonly compared with the llama or the alpaca but different from goats (4 mL/kg/min).9,10 This suggests that extrapolation of drugs eliminated via renal filtration may have different elimination rate kinetics in llamas and alpacas compared with those in ruminant species. Little is known about hepatic metabolic processes in llamas or alpacas besides data from case reports on hepatic lipidosis, triglyceridemia, and fascioliasis. The veterinarian should, however, assume that drug metabolism will not be exactly the same as in other species, and specific knowledge of pharmacokinetics and metabolism in llamas and alpacas is highly desirable to make drug decisions.
Toxicity of drugs may differ among species because of pharmacokinetic or pharmacodynamic considerations. Although reports of toxicity of pharmacotherapeutics in llamas or alpacas are limited, one example is a report of albendazole toxicosis in alpacas.11 In this case, the dose used was the dose recommended for ruminant species (10 mg/kg), and clinical signs and death resulted from this use. This report points to the fact that adverse reactions may not be predictable and that safe doses are not always the same across purportedly similar species.
Anatomic features, rather than physiology, may affect selection of route of administration of drugs: Llamas and alpacas have anatomic idiosyncrasies when it comes to accessing veins for drug delivery.3 Although venous access is not prohibitive, not all facilities, animal personalities, nor technical expertise allow for easy access to veins for intravenous (IV) administration of drugs. Certainly in ruminant species, IV administration may not be particularly easy because of facility and restraint requirements, but anatomically, ruminants tend to be easier when it comes to administering IV medications, and certain drugs achieve more desirable pharmacokinetic profiles (e.g., some antimicrobial drugs) or cause less tissue damage when administered intravenously (e.g., flunixin meglumine).
Another anatomic feature that may impact drug use is the fact that weight estimation in these species may be inaccurate because of the presence of fiber coat. It is important for veterinarians selecting dosages to accurately estimate or actually measure the weight of these animals to prevent inappropriate dosing—either underdosing, which can result in inefficacy, or overdosing, which might result in toxicity. These species are often also overfed, at least in the United States, so body condition scoring (BCS) may be appropriate in addition to measuring weight.12 Whether lean body mass should be used to dose drugs is debatable but might need to be considered as recommended in other species.
Physiologically and anatomically, llamas and alpacas are very similar except for mature size, so it has been suggested that they be considered equivalent in terms of drug approval and that if drugs were to be approved in the United States for one species, the other should be automatically included.1 Limited reports comparing the two are available, and the pharmacokinetic behavior has been studied for only a few drugs. Two reports on parasiticides studied llamas and alpacas simultaneously: When moxidectin was administered, neither species attained quantifiable concentrations to enable comparisons to be done.13 However, when doramectin was administered, pharmacokinetic parameters such as Cmax and area under curve (AUC) were similar between the two species, although no statistical analysis was done.14 This hints that drug disposition is expected to be similar between llamas and alpacas, but more data are needed to be conclusive.
Clinical Setting
This companion or pet animal status of most llamas and alpacas in the United States means that they are usually not considered food animals and thus are not likely to enter a federally inspected slaughter facility. (The U.S. Department of Agriculture and the Food and Drug Administration Center for Veterinary Medicine still might classify them as food animals, so discussions with a federal regulator might be appropriate in case of any concerns.) Since they are not considered food animals, the restriction on drugs as listed in the Animal Medicinal Drug Use Clarification Act of 1994 and subsequent regulations do not apply.15 In effect, the following drugs are permissible for use in llamas and alpacas: metronidazole, clenbuterol, chloramphenicol, phenylbutazone, nitrofurans, fluoroquinolones, and glycopeptides.
Review of the Principles of Drug Selection
Principles of Reviewing for Evidence
To provide the best veterinary care, veterinarians must make decisions using the best evidence. This is the main tenet of evidence-based veterinary medicine (EBVM), which is the process of integrating best research evidence with clinical expertise and owner and manager values.16 Clinical expertise develops during professional training, continuing education, and clinical experience; owner and manager values are gauged at the time of decision making by the judgment of the attending veterinarian. But what about finding the best research evidence? Many veterinarians feel uncomfortable with the skills needed to find evidence, or they are not convinced that research evidence is necessary to make clinical decisions. Although convincing veterinarians to apply research evidence to their daily practice is not the goal of this chapter, discussing the skills of finding and reviewing evidence are addressed here.
The basic steps of practicing EBVM are shown in Figure 34-1.17 The first step guides the search for evidence and is an important part of the process. A question that is too broad cannot be answered, and a question that is too specific will not uncover any evidence. An easy way to create a clinical question is using the PICO format: Patient, Intervention, Comparison, and Outcome. For example, a question might be: “For crias with failure of passive transfer, does administration of llama plasma increase survival rate?” The second step may seem difficult to the uninitiated but, in fact, can be performed quite readily on free medical literature databases such as MedLine or with the assistance of veterinary or health sciences librarians at your alma mater or closest veterinary or medical college. (Several veterinary organizations are beginning to provide access to certain fee-based resources as part of membership, e.g., American Association of Equine Practitioners.) Another source of guidance is the series of articles begun in the Journal of the American Veterinary Medical Association in late 2009, called “What’s the Evidence?”18–22 Each article demonstrates the use of evidence-based principles in actual clinical cases, including discussions of evidentiary search strategy and final decisions made.
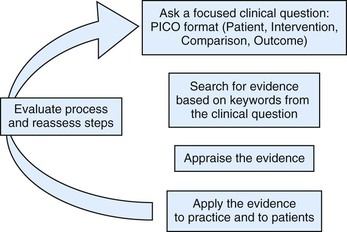
Figure 34-1 Steps in practicing evidence-based veterinary medicine. (From Cockcroft P, Holmes MA: Handbook of evidence-based veterinary medicine, Oxford, U.K., 2003, Wiley-Blackwell.)
The third step of evaluating the evidence has been discussed at length in numerous publications, with advice about how to evaluate statistics, how to look for bias, and so on. These are important skills for those delving into the literature, but the practicing veterinarian may start with a basic assessment of the level of evidence a particular source provides. A number of schemes for assigning a level exist, but one in particular was developed by veterinary pharmacologists and staff at the U.S. Pharmacopeia, the international drug standard setting body, with the types of research conducted in veterinary species in mind.23 Those levels are listed in Box 34-2. If veterinarians can make the distinction between a randomized trial and a case series, with the latter being lower on the evidence scale, we would go a long way toward making decisions based on better evidence. This scheme for assigning evidence levels is used in the discussion and tables in this chapter about what has been published on pharmacotherapeutics in llamas and alpacas.
Principles of Extrapolating
To determine doses of drugs for llamas and alpacas, many clinicians use the doses reported in ruminant species such as sheep, goats, or cattle, or in other large animal species such as horses. The accuracy of such uses has not been systematically compared, but a short discussion is warranted. The differences in adult size as well as in physiology and anatomy from the ruminant or equine species suggest that when selecting doses for llamas and alpacas, a more methodic approach that involves not simply scaling by weight but by considering factors that are independent of size and weight might be appropriate.24 Allometric scaling is one example of a methodology for scaling across species, which is based on how biologic parameters vary with animal size.25 Metabolic rate, one biologic parameter, has experimentally been determined to vary in proportion to body weight to the exponent 0.75, that is, mathematically denoted as BW0.75. It would be attractive if drug disposition parameters such as elimination half-life also varied in proportion to Wx, but this has not always been borne out in analyses of pharmacokinetic data.26 However, using allometric scaling of pharmacokinetic parameters from other species is labor intensive and requires access to pharmacokinetic data from multiple species and plotting it against body weight, which is beyond the scope of this chapter.
Therefore, in the absence of allometric scaling data, pharmacokinetic data could be compared for llamas and alpacas and reasonable approximations from related species (cattle, sheep, goats, horses, camels) could be used to determine which species are most similar and from which currently available formulary dose extrapolations can be made. In particular, pharmacokinetic data from camels are attractive, since camels and llamas and alpacas are evolutionarily and anatomically related, and one might assume that drug disposition might be similar among those species.27,28 In addition, a number of reports of pharmacokinetic data in camels have been published, in particular because of the racing camel industry in the Middle East. However, comparisons of clearance between camels and llamas or alpacas for caffeine, ceftiofur, and enrofloxacin suggest the values are not similar enough across drugs to recommend extrapolating from camels more than any other species.29–35 Comparisons between cattle and llamas or alpacas for pharmacokinetic parameters for intramuscular ampicillin sodium, enrofloxacin, and gentamicin (although formulation differences may exist, and differences may be caused by rates of conversion to ciprofloxacin) also revealed no obvious trend in similarities.34,36–42
Principles of Antimicrobial Drug Therapy
Amount of Drug Necessary to Inhibit Growth.
The thresholds that determine the categorization into susceptible or resistant are best represented by those published by the Clinical and Laboratory Standards Institute (CLSI), an international, interdisciplinary, standards-developing, and educational organization that promotes the development and use of voluntary consensus standards and guidelines within the health care community. The CLSI standards for antimicrobial susceptibility testing are published and used by veterinary diagnostic laboratories and scientists in the United States and around the world.43 The thresholds are delineated in interpretive criteria, that is, the inhibitory concentrations that should be used to identify susceptible or resistant isolates. For example, the interpretive criteria for gentamicin in horses for Enterobacteriaceae, Pseudomonas aeruginosa, and Actinobacillus spp. are that organisms with MICs 2 mcg/mL or less should be called “susceptible,” with MICs 4 mcg/mL should be called “intermediate,” and with MICs greater than or equal to 8 mcg/mL should be called “resistant.” The interpretive criteria provide the translation from in vitro concentrations to in vivo prediction of clinical efficacy, and they are based on pharmacokinetics data, pharmacodynamics data, MIC data from many wild-type bacterial isolates, and clinical trial data, if available.
Because interpretive criteria have not been evaluated or validated for llama and alpaca pharmacokinetic data, these criteria must be viewed with circumspection, and the resultant report from the laboratory stating “susceptible” must be viewed with caution in terms of its ability to predict clinical success. It is also important to note that interpretive criteria have not been validated for any species for topical therapy, for intraocular therapy, or for organisms that cause disease within the gastrointestinal tract.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree