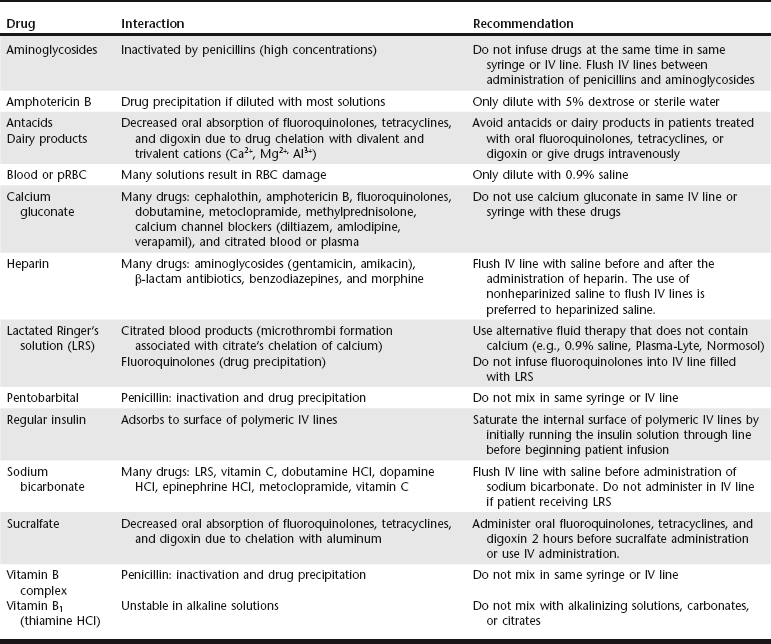
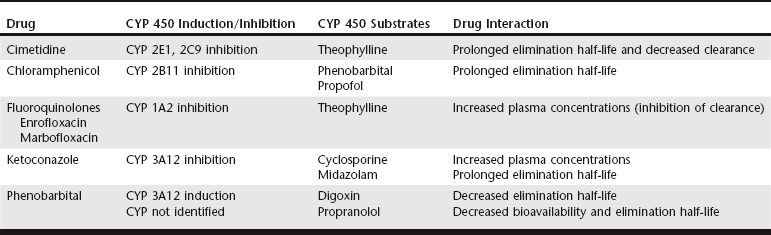
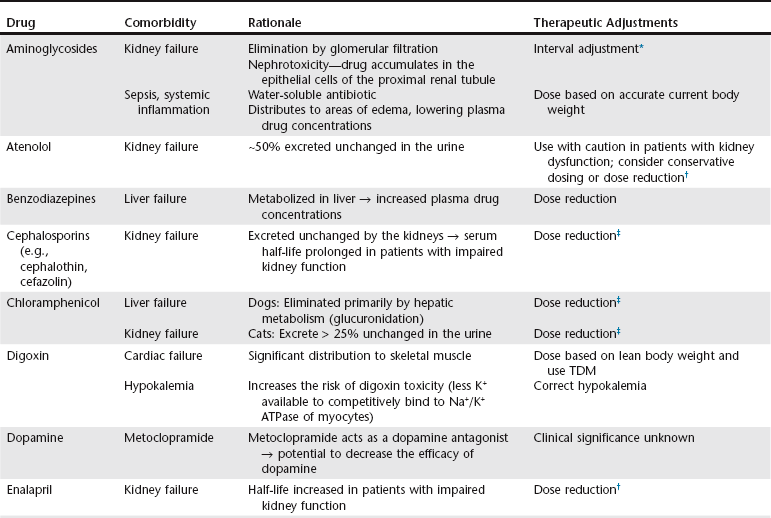
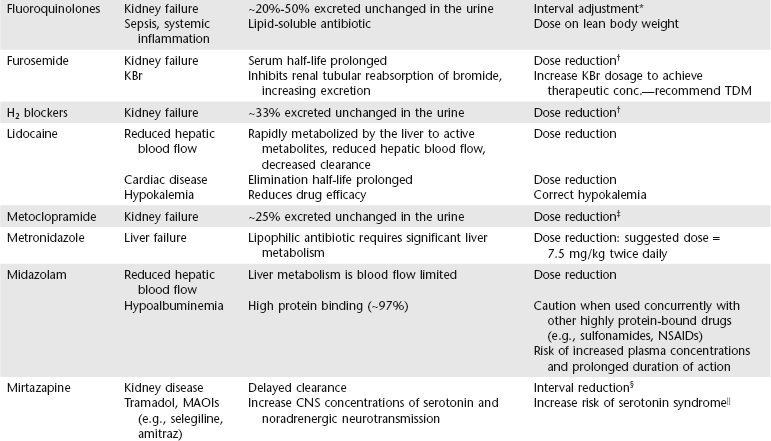
Chapter 6 An ADE is broadly defined in human medicine as “any noxious, unintended, and undesired effect of a drug, which occurs at doses used in humans for prophylaxis, diagnosis, or therapy… [This excludes] therapeutic failures, intentional or accidental poisoning or drug abuse, and adverse effects due to errors in administration or compliance” (Bowman, Carlstedt, and Black, 1994). These ADEs can be dose-dependent (predictable) or dose-independent (unpredictable) and are relatively common in human ICUs, reported at approximately 29.7 per 100 ICU admissions (WHO, 2000). There are no data available about the incidence of ADEs in veterinary ICU patients. Drug administration is a key part in the treatment and management of ICU patients. The clinician can minimize ADEs and maximize medication efficacy by considering both the pharmacologic properties of the drugs being administered and patient characteristics that can modify drug effects. Factors that contribute to ADEs can be divided into two categories: drug-related factors and patient-related factors. Drug-related factors refer to the basic physical and chemical incompatibilities that exist when certain drugs are combined. These most commonly occur when drugs are given in succession through an intravenous catheter or fluid delivery line and can be anticipated (Table 6-1). A less appreciated drug-related factor that can contribute to ADEs is the formulation. For example, long-acting drug formulations such as methylprednisolone acetate, triamcinolone acetonide, and cefovecin can persist in the body for weeks, eliminating the option of drug withdrawal if side effects develop. In addition, long-acting glucocorticoids do not allow a controlled dose taper and long-acting antibiotics increase the risk of developing antimicrobial resistance. More difficult to predict are those patient-related factors that predispose to ADEs. ICU patient populations at increased risk for ADEs include those that are dehydrated or have decreased total body water, receiving multiple concurrent medications (polypharmacy), or that have altered organ function (Table 6-2). In addition, the very young and elderly patient populations are at increased risk for ADEs. A number of examples can be cited and are the main focus of this chapter. TABLE 6-2 Drug Interactions and Suggested Therapeutic Adjustments in Commonly Used Drugs in Critically Ill Patients *Consider interval adjustment for concentration-dependent antibiotics: †New dose = Standard dose × (Normal serum creatinine / Patient’s serum creatinine). ‡Consider dose adjustment for time-dependent antibiotics: New dose = Standard dose × (Normal serum creatinine / Patient’s serum creatinine). §Mirtazapine 1.88 mg/cat every 48 hours is recommended dose in cats with chronic kidney disease (Quimby et al, 2011). The major phase I metabolic machinery of the liver is the cytochrome P450 (CYP 450) enzyme system. Drugs can either inhibit or induce CYP 450 enzymes and result in clinically significant drug-drug interactions if coadministered. Additionally, cholestasis can sometimes decrease the activity of CYP 450 enzymes in the liver. Table 6-3 summarizes some of these potentially significant drug interactions based on CYP 450 enzyme inhibition or induction.
Drug Incompatibilities and Drug-Drug Interactions in the ICU Patient
Adverse Drug Events
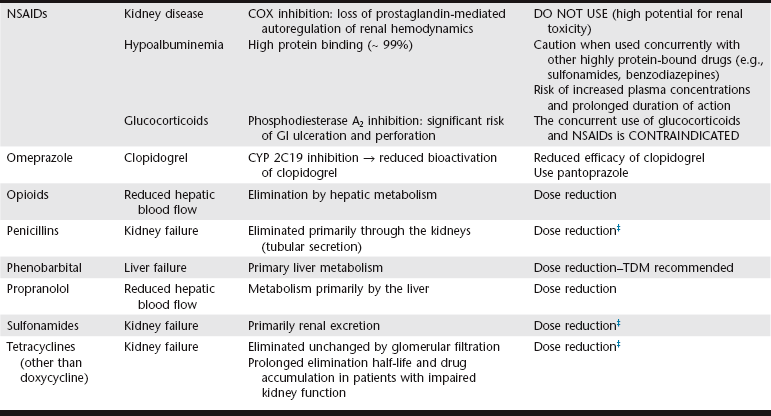
New interval = Standard interval × [1 ÷ (Normal serum creatinine / Patient’s serum creatinine)]
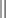 Clinical signs consistent with serotonin syndrome include agitation, tachycardia, hyperthermia, mydriasis, muscle twitching, vomiting, or diarrhea.
Clinical signs consistent with serotonin syndrome include agitation, tachycardia, hyperthermia, mydriasis, muscle twitching, vomiting, or diarrhea.
Liver Disease
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree