Disorders of the Urinary System
Camelid kidneys are reniform and not grossly lobulated. Ureteral and bladder anatomy is conventional. The female urethra is also conventional and exits through the urethral tubercle on the dorsal aspect of the suburethral diverticulum. The penile urethra is long and narrow. Male camelids have a dorsal urethral recess at the ischiatic arch and a sigmoid flexure near the scrotum.1 The tip of the penis is contained within fused folds of the prepuce until sexual maturity and may remain so entrapped in early castrates. In most intact male camelids, the preputial attachments break down when they are between 1 and 2 years of age.
The most common blood values used to assess the kidneys are urea nitrogen and creatinine (Box 39-1). Urea nitrogen is made in the liver to detoxify the ammonia produced during amino acid catabolism. It is excreted through urine but also moves freely through most body compartments. Gastrointestinal (GI) microbes may use urea as a nonprotein nitrogen source, so during short periods of anorexia, blood urea may fall because of microbial consumption. During longer periods of anorexia, when a die-off of gastric microbes occurs, blood urea may rise slightly.
The kidneys also help maintain electrolyte and acid–base homeostasis. Camelid urine usually is alkaline and has low concentrations of sodium and chloride and a high concentration of potassium (see Box 39-1). Abnormalities such as a malformation, ruptured ureter, or leaking bladder, which result in return of urine to the extracellular space, may result in systemic alkalosis, hypochloremia, and hyponatremia. Changes in blood potassium are variable because urinary disorders frequently are accompanied by anorexia and decreased dietary potassium intake. Changes in calcium and phosphorus are also variable and depend on diet and vitamin D status. Azotemia is often accompanied by hypermagnesemia.
Camelids tend to drink less and urinate less compared with ruminants, potentially because of greater GI conservation of water.2 Urine output ranges from about 5 to 7.5 milliliters per kilogram per day (mL/kg/day) in adults. Fractional excretions of electrolytes and activities of enzymes in urine have been determined and do not differ substantially from ruminants fed similar diets (see Box 39-1).2 However, obtaining urine may be problematic. Free-catch samples may be obtained from a distance using a cup on a stick, and females may be catheterized with practice. Narrow, semirigid tubing works best. Males may also be catheterized using a 3 to 5 French (Fr) urinary catheter, if the penis can be extruded.3 Cystocentesis is difficult unless the bladder is grossly distended and may lead to hemorrhage or urine leakage. Once obtained, urine is analyzed using conventional techniques. Camelid urinalysis values are similar to those of other species except that glycosuria is common (see Box 39-1).
Plain-film radiography may be used to find mineralized densities or to identify a grossly distended bladder, ureter, or kidney. Contrast material may be injected in a retrograde fashion through the tip of the penis, through the urethral tubercle, or through a cystotomy tube to highlight structures. Five to 10 mL of contrast material is sufficient to outline the normal urethra, whereas up to 50 mL in a cria and 200 mL in an adult may be necessary to highlight the entire bladder. Excretory urography may be used to examine renal uptake, the ureteral structures, and the filling of the bladder in crias and alpacas but is practically difficult in adult llamas. For the excretory urogram, we have administered 75 mL of diatrizoate meglumine and diatrizoate sodium (Renocal 76) intravenously (IV) to crias and 200 mL to adult alpacas and taken abdominal images at 5-minute intervals for up to 30 minutes. If the bladder or the bladder filling is the primary region of interest, 45- and 60-minute images may be taken as well. Computed tomography (CT), nuclear scintigraphy, and other cross-sectional imaging modalities have seen limited use in camelids, but their use is increasing as these modalities become cheaper and more widely available.4,5
Parts of the urinary tract may be visually examined by means of laparoscopy.6,7 From the right paralumbar fossa, the right kidney is usually visible, although it may be obscured by retroperitoneal fat; on one occasion, the kidney was lacerated.6 The bladder is sometimes visible from this site. From the left paralumbar fossa, the left kidney is visible about half the time.7 The kidneys and the bladder are inaccessible from a ventral approach. Direct visualization during conventional surgery is also possible.
Clinical signs of urinary tract disorders vary with the nature of the disorder. Obstructive or inflammatory lesions often lead to signs of pain, including stretching, restlessness, lying in an abnormal posture, reluctance to stand, inappetence, urinating in small spurts, dribbling of urine, frequently posturing to urinate, and stranguria (Box 39-2). Lesions associated with loss of renal parenchyma or reabsorption of urine often lead to anorexia and obtundation related to the degree of azotemia. Inflammatory or neoplastic lesions often lead to changes in the nature of urine, with visible increases in turbidity or redness, associated with protein or blood loss in urine (Box 39-3). Disorders associated with internal urine leakage will furthermore lead to subcutaneous swelling or abdominal distension.
Congenital Lesions
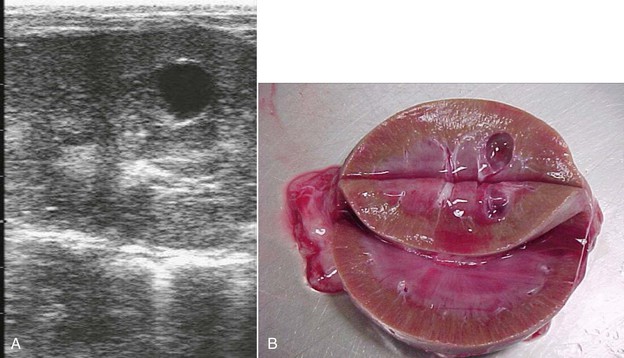
Malformations of the kidney include parenchymal cysts (Figure 39-1), horseshoe kidneys, dysplasia, and agenesis (Figures 39-2 and 39-3).8–11 Lesions may be unilateral or bilateral. Abnormal kidneys may be absent, small, or enlarged and saclike. The more normal, contralateral kidney, if present, often undergoes compensatory hypertrophy. Agenesis and horseshoe kidneys often occur in conjunction with atresia ani or other urogenital lesions but may also occur as solitary lesions. Clinical signs depend on the amount of functional renal tissue. Unilateral abnormalities may be clinically silent and only discovered postmortem. With bilateral renal agenesis, signs of lethargy, anuria, and mild abdominal distention often begin within 24 hours of birth and progress over 3 to 4 days. With unilateral agenesis, signs may remain absent, unless some pathologic process affects the remaining kidney. Dysplasia and cystic lesions may lead to signs in juvenile camelids or remain silent for several years. Cysts appear to grow with time and may represent a milder form of the process causing agenesis.12 Urinalysis may be normal or, in severe cases, may reflect failure to concentrate and proteinuria. Anuria is expected only with bilateral agenesis or severe bilateral disease from one of the other causes. Blood abnormalities also reflect the severity of the conditions. BUN and creatinine concentrations may be normal with mild, unilateral, or early disease and show steady increases with clinical disease. As with other renal causes of azotemia, the BUN-to-creatinine ratio (in mg/dL) is frequently much lower than the 20:1 expected with prerenal azotemia.
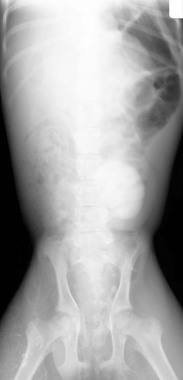
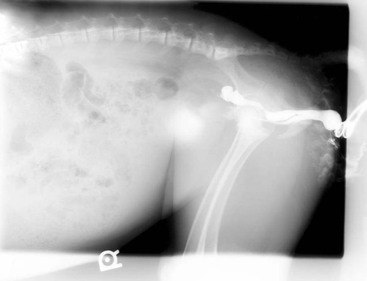
Figure 39-2 Excretory urogram of an alpaca cria with unilateral renal agenesis and urine dribbling. Atresia ani had been repaired previously. Note the lack of contrast uptake or any identifiable renal structure on the right side (left side of image). The left kidney appears to be functioning normally.
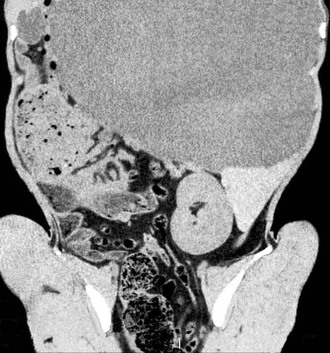
Figure 39-3 Computed tomography image of an adult alpaca with right renal agenesis. Compare in appearance to Figures 39-9 and 39-10, which show both kidneys clearly.
In larger camelids, primarily with unilateral malformation, an absent or enlarged left kidney may be palpated per rectum. Ultrasonography may reveal hydronephrosis, abnormal conformation, the presence of cysts (see Figure 39-1), or lack of functional renal cortical tissue. The less affected or unaffected kidney is frequently grossly enlarged and may also be visible on plain abdominal radiography. Various other imaging modalities such as contrast radiography (see Figure 39-2), cross-sectional imaging studies (see Figure 39-3), or nuclear scintigraphy may reveal the lack of renal tissue or function. With bilateral disease, the bladder may remain empty and appear to be absent as well. Biopsy may be used to confirm dysplasia but is frequently unnecessary for other types of lesions. Kidneys with poor drainage also appear to be prone to developing infections, so biopsies and fluid samples may reveal bacterial inflammation and pyuria. Unilateral lesions may respond to nephrectomy of the affected kidney, which should be considered if the opposite kidney appears visibly and functionally normal and the animal displays persistent azotemia, proteinuria, hematuria, or infection associated with the abnormal kidney.4,11,13 Some camelids with unilateral renal agenesis or severe dysplasia have lived to thrifty adulthood, with the renal abnormality found incidentally after death. Similar congenital renal abnormalities have been found in closely related camelids.
Congenital malformations of the ureters include ectopic ureters, atresia, and ureteral duplication.4,8,10,14 Atresia leads to rupture and retroperitoneal urine accumulation, resulting in poor growth, progressive lethargy, possible abdominal discomfort with straining, and slow accumulation of abdominal fluid in young crias. Ureteral duplication is similar, with a functional and nonfunctional ureter contained within the same sheath from one kidney. Signs may progress over several weeks to months because the functional kidney is somewhat able to compensate. Signs may also wax and wane. Urinalysis is usually normal, but blood evaluation may reveal renal azotemia. Ultrasonography reveals diffuse edema throughout the dorsal lumbar retroperitoneal space. Megaureter and hydronephrosis may develop and may be detected by ultrasonography, radiography, or other imaging studies. Excretory urograms or scintigraphy may be used to highlight the lesions. An increased amount of abdominal fluid may be detected. Given the size of the animals affected, endoscopic examination of the bladder would be difficult but could potentially reveal a lack of urination through a dysfunctional ureter. If the lesion is unilateral, nephrectomy may be curative.4,13
Ectopic ureters frequently lead to urinary incontinence, often with associated tail, perineal, and hindlimb skin scald. Except for discomfort from the skin lesions, and mild stranguria with infections, the camelid frequently appears healthy. As such, camelids of any age may be seen with this disorder, but signs should have been noticed shortly after birth. Unless a secondary bacterial infection exists, which is common with lesions of this nature, blood and urine evaluation should be normal. Ectopic ureters may rejoin the urogenital tract at almost any point or enter the terminal intestinal tract, causing diarrhea. Endoscopic evaluation of the vaginal vault, suburethral diverticulum, urethral tubercle, urethra, and bladder and contrast-enhanced CT have been used to define the nature of the lesion.4 Ultrasonography may reveal an abnormal, hydronephrotic, or infected kidney and hydroureter, but the ureter itself is difficult to follow to its final destination. Contrast radiography may be attempted in younger crias. Because of the chance for ascending infections and the persistent skin infections, unilateral nephrectomy is recommended and may be curative for unilateral lesions.4,13
A number of abnormalities of the terminal urinary tract are related to malformations of the genital tract (Figure 39-4).8,14–20 These include urethral duplication and various malformations of the penis in hermaphrodites or other sexually ambiguous animals and terminal obstruction with labial fusion or vulvar hypoplasia. These may result in anuria, dysuria, or stranguria and frequently are accompanied by visible or palpable abnormalities of the external genitalia. With complete obstruction or gross stenosis, fluid-filled cystic structures may appear at the vulvar opening in presumptive females or along the course of the penile urethra in presumptive males. Occasionally, signs are interpreted as tenesmus, and a history of laxative or enema administration may exist. Mild to severe azotemia and evidence of bladder, ureteral, or renal distention may be present, depending on the severity of the obstruction. In females, some urine may accumulate in the uterus as well. Contrast radiography is especially useful in defining the presence, appearance, and function of various structures. Relatively simple surgical interventions may restore the flow of urine, but the overall implications of a morphologically abnormal and potentially genetically ambiguous animal should not be ignored. Vulvar fusion or hyperplasia in closely related animals has been reported in the scientific literature and anecdotally.17 A similar disorder has been reported in camels.21
Acquired Urinary Tract Obstruction
Acquired urinary tract obstruction is one of the most debilitating disorders of male camelids because their anatomy complicates all attempts at correction.3,14,22–26 Gelded males, especially those gelded at a young age and presumably retaining a narrow urethra, appear to be at greater risk than intact males. The long, narrow urethra of male camelids may make them even more prone to obstruction than other species under similar conditions. Camelids’ low urine output may further contribute to the obstruction. Male camels are similarly affected.27 Females, which have a wider, shorter urethra, are generally spared, although they occasionally develop obstruction from ureteroliths, pelvic displacement of the bladder, or severe cystitis.28
Uroliths and debris from cystitis or urethritis are the most common causes of acquired urethral obstruction.3,14,24–26 Other possible causes include pelvic displacement of the bladder, urethral tumors, hematoma, tumor or abscess within the pelvic canal, upper motor neuron disease, infection of accessory sex glands, cystic mucosal lesions, stricture at a previous obstruction or surgery site, congenital malformations, bladder polyps, and cystitis.14,28–31 Other factors that may contribute to obstruction include trauma to the tip of the penis, reduction in urine volume because of reductions in water intake or water content of feed, dietary mineral imbalances that promote excretion or aggregation of urinary mineral, dietary oxalates, and factors that increase the mucoproteinaceous content in urine. Birthing trauma is associated with intrapelvic hematomas and abscesses in females, and Streptococcus equi ssp. zooepidemicus may cause lymph node abscesses in any age or gender of camelid. Vitamin A deficiency, although not verified in camelids, may contribute by promoting exfoliation of the bladder mucosa.
Most intraluminal obstructions occur within the last 6 cm of the penis, although not necessarily at the tip. The sigmoid flexure is the next most common site, followed by the trigone region and pelvic urethra. In rare cases related to renal disease, the lith or debris obstructs the ureter or renal pelvis. Occasionally, a single, large lith causes the blockage, but more commonly, several centimeters of urethra are filled with small, gritlike stones and inflammatory debris. In the Pacific Northwest, the most common liths are silicates, with calcium carbonate, calcium oxalates, and calcium hydroxyapatite also found.3,26 Struvite stones are less common but have been seen. Stones of mixed composition are common. Silicate crystals are associated with mature forages, which contain up to 8% silicon compounds. These are concentrated and polymerize in urine, where aggregation with protein leads to liths. Calcium is also concentrated in urine when overfed or in vitamin D-deficient animals. Struvite crystals, rare in camelids, are usually caused by overfeeding calcium, magnesium, and phosphorus in grains. When liths are involved, multiple animals on a farm may become affected over a period of several months. The occurrence of uroliths appears to vary regionally, with the Pacific Northwest of the United States a higher prevalence area than the front range of the Rocky Mountains. Uroliths have been reported in camelids as young as 3 months of age.26
In about half of the cases of intraluminal obstruction seen at our clinic, no stone is found. Some of these camelids have mucoproteinaceous plugs, consisting of fibrinonecrotic material. A case with a similar finding was reported in the literature, and as our experience with camelids grows, it is becoming more apparent that many urethral obstructions are potentially a consequence of prostatitis or cystitis.22 A variety of organisms have been cultured from the urine or urinary debris of these animals, including Escherichia coli, Klebsiella, Streptococcus, Pseudomonas, and Arcanobacter pyogenes. It is possible that camelids are more prone to developing obstruction after cystitis compared with other species, most likely because of the narrow camelid urethra.
Displacement or eversion of the bladder into the pelvic canal is an uncommon occurrence, reported once in the literature and twice anecdotally.28 All cases were females, but only one was of breeding age and was close to parturition. Accidental breeding was not considered likely in the other two animals. Hematuria may be present but is not marked, and catheterization of the bladder is difficult to impossible. Transrectal palpation or ultrasonography may give the impression of the fluid-filled viscus in the canal, but excretory urography (or retrograde cystography, if the urethra is patent enough) is necessary to demonstrate the body of the bladder everted dorsocaudal to the trigone and within the pelvic canal.
A congenital malformation associated with acquired urinary tract obstruction is urethral duplication.15 With this abnormality, the duplicated remnant within the pelvic canal intermittently fills with urine, physically compressing the patent urethra. Thus, signs may not be present at birth and may wax and wane over many weeks. Urethral duplication is best identified with contrast studies, which allow careful evaluation of multiple structures and their connections.
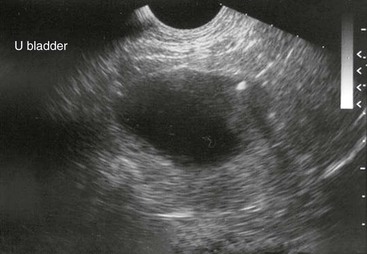
With urine leakage into tissues or the abdomen, signs of lethargy, weakness, and anorexia begin to supercede pain signs. Visible or palpable edematous periurethral tissue or abdominal distention may be present. Transrectal or transcutaneous ultrasonography may reveal a distended urethra and bladder, potentially with shadowing areas around mineralized densities (Figures 39-5 and 39-6). Mineral densities may be seen swirling within the bladder. In the case of intraabdominal urine leakage, increased free abdominal fluid may be imaged. Hydroureter and nephrosis may be present in camelids with longstanding obstruction. Transrectal ultrasonography is also an effective means of identifying tumors, abscesses, hematomas, and prostatic or cystic abnormalities. Radiography may reveal the larger, calcium-containing stones, but without contrast, it is not reliable for demonstrating sabulous obstructions, silicates, or mucoproteinaceous plugs (Figure 39-7). Radiography, especially with contrast in place, may reveal intrapelvic displacement of the bladder. Endoscopy of the bladder is helpful for identifying tumors, polyps, or cystitis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree