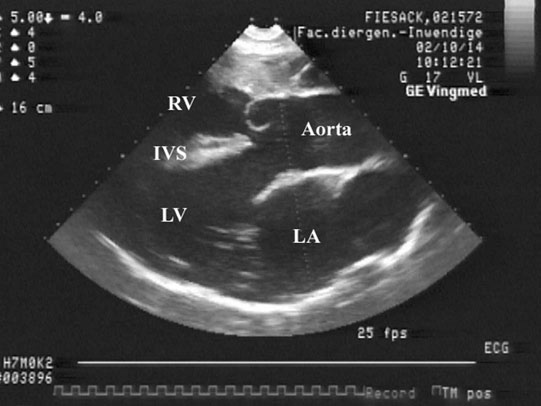
• Clinical signs. Auscultation and cardiac echocardiography may demonstrate a PDA or persistent foramen ovale. • Following O2 administration, a right foreleg (preductal) PaO2 will likely show a tension difference/gradient (> 20 mmHg) when compared to a hind limb (postductal) if PDA shunting is substantial. This gradient may not be present, however, if foramen ovale shunting is marked. • Treatment generally needs to be undertaken at a referral institute and may include mechanical ventilation, decreasing pulmonary hypertension by the use of inhaled nitric oxide or sildenafil. • Foals in which suspicion that the PDA is functionally patent for greater than 1 week should have further cardiac diagnostic testing with echocardiographic +/− radiographic and blood gas evaluations. • Since the patency of the PDA is maintained by production of PGE2 by the ductus arteriosus, prostaglandin suppression through cyclo-oxygenase inhibitors (i.e. flunixin meglumine, ibuprofen, indometacin, etc.) may elicit closure of the PDA in some cases. • VSDs are usually associated with at least two murmurs. The shunt causes an obvious, loud, pansystolic murmur in the area of the aortic and tricuspid valves. The persistent, undulating, ‘machinery type’ murmur has a marked precordial vibration (thrill) which radiates widely and is audible on both sides of the chest, but is usually significantly louder on the right. The thrill can often be appreciated merely by palpation of the lower right thoracic wall. This murmur usually becomes more prominent with exercise and rising blood pressure. There is also frequently a second murmur associated with relative pulmonic stenosis. Although the right ventricular tract is normal there is an increased volume of blood leaving the right ventricle which results in a loud holosystolic crescendo–decrescendo murmur over the pulmonic valve in the left third intercostal space. This murmur is usually at least one grade lower than the VSD murmur. • Peripheral signs of circulatory status and electrocardiographs are usually normal. • Ultrasound studies including Doppler are the best diagnostic tools as they not only determine the presence and size of the defect and the shunt velocity but can also be helpful to assess the hemodynamic burden. • Major septal defects, which shunt significant volumes of blood into the relatively lower-pressured right side, result in gross right-sided enlargement and this may be detected on percussion or, more precisely, by radiographic and ultrasonographic examination of the chest. • Membranous defects that are smaller than 25 mm and demonstrate a shunt velocity of greater than 4 m/s may be exercise-tolerant. • In smaller breeds of horses and ponies it is also useful to compare the size of the VSD with the diameter of the aortic root. Those which are less than one third of the aortic root carry a better prognosis. • Muscular ventricular septal defects, defects with aortic insufficiency, or other anomalies are likely to be intolerant of strenuous exercise. • Foals with membranous VSD larger than 35 mm in diameter or muscular VSDs tend to have decreased life expectancy. VSD may also be a component of complex cardiac anomalies carrying a poor prognosis. • Treatment of significant defects is generally impractical and owners should be advised against using the horse for athletic purposes or breeding. • Most cases are obvious in the first few weeks of life with a pronounced heart base murmur and are frequently exercise-intolerant at presentation. • Clinical signs of right-sided volume overload predominate (jugular pulsation, ascites, pleural effusion, peripheral venous distension and edema) and supraventricular arrhythmias (especially atrial fibrillation) are not uncommon due to right atrial enlargement. • Definitive diagnosis is made with echocardiography. Color flow Doppler may be required to differentiate a residual, non-functional foramen ovale from a true ASD. • No treatment is usually undertaken as the prognosis is poor. • Clinical signs are diagnostic. A loud grade 4/6 to 6/6 pansystolic murmur, with palpable thrill over the left heart base, with point of maximal intensity (PMI) over the pulmonary valve area. The defect may also be audible on the right side but is usually less intense on this side. • Radiographic examination of the thorax will usually identify the grossly enlarged right ventricle and a very prominent ascending aorta. • Two-dimensional echocardiography demonstrates echo banding in the tricuspid valve region, fallout of the aortic root, enlarged left ventricle, small right ventricle, and variable atrial enlargement and mitral excursion variations. • Contrast echocardiographic techniques confirm intracardiac shunting of blood from right to left atrial chambers then to left ventricle if shunting is suspected. • On auscultation it is classically described as an ‘irregularly irregular’ rhythm. • Atrial fibrillation is easily identified on an ECG strip as multiple rapid atrial ‘f’ waves preceding regular QRS complexes. In horses with sustained atrial fibrillation an echocardiogram should be performed to determine whether there is any underlying cardiac disease. • Laboratory studies for determination of electrolyte abnormalities may be useful for horses with paroxysmal atrial fibrillation. • Therapy with β-blockers, class 1A antiarrhythmics (quinidine, procainamide, etc.), class 1C (flecainide, etc.), class III medications (amiodarone, etc.) and electroconversion should only be reserved for intractable or persistent atrial fibrillation (greater than 48 hours duration) without spontaneous conversion to normal rhythm or with clinical signs of exercise intolerance, lethargy, etc. • Auscultation reveals a regular sinus rhythm that is interrupted by an obviously premature beat. Confirmation is by electrocardiogram (ECG). Documentation during exercise may be required to demonstrate a relationship with poor performance. • Therapy is not often recommended unless atrial tachycardia arises. Atrial tachycardia is defined as more than four consecutive premature atrial beats. Transient atrial tachycardia can occur in structurally normal hearts during exercise, catecholamine release, hypoxia, hypovolemia, electrolyte alterations and caffeine therapy due to increased automaticity or triggered activity. • Quinidine or procainamide can be considered but are impractical for long-term use. Digoxin is an alternative therapy but its effectiveness in such cases has not been evaluated in large studies. • Other considerations of therapy are maintenance of normal serum potassium levels and treatment of any underlying cardiac disease such as myocarditis. • Early beats can be heard via auscultation and pulse deficits are common. • ECG findings reveal lack of p waves preceding wide and/or tall (‘bizarre’) QRS complexes. If the QRS complex morphology varies, these VPCs are considered polymorphic and are assumed to arise from multiple/variable foci within the ventricles indicative of more diffuse myocardial disease. • A pronounced jugular pulse with the VPC may be observed due to atrioventricular asynchrony (cannon A wave) and the follow-up beat after a VPC may be stronger due to the increased left ventricular filling that occurs with a compensatory pause following the VPC. This is known as extrasystolic potentiation leading to greater intensity of this beat. • A physical examination, complete blood count, serum chemistry, blood gas (arterial +/− venous), echocardiogram and ECG evaluations should be considered to define the inciting cause(s). • Evaluation of electrolyte abnormalities, as well as measurement of ionized calcium and magnesium, is recommended. Hypokalemia and hypomagnesemia are frequent causes of VPCs and electrolyte derangements may affect the usefulness of specific antidysrhythmic agents such as lidocaine. • Measurement of cardiac Troponin 1 and other cardiac enzymes can be used to document underlying cardiac disease; 95% of normal healthy foals in one study had cardiac Troponin 1 values <0.49 ng/ml. Values ≥1 ng/ml are highly suggestive of myocardial disease/inflammation. • The majority of horses with VPCs will have spontaneous resolution of the arrhythmia after 4–8 weeks of rest. • Patients with abnormal VPCs should be closely monitored such that ventricular tachycardia (VT) does not arise, i.e. heart rate >100–120 beats per minute with the rhythm being initiated from below the bundle of His (ventricular origin). Although potassium imbalance (both hypo- and hyperkalemia), other electrolyte disorders (hypomagnesemia), toxicants, vitamin E/selenium deficiency and stimulants can lead to VT, primary myocardial disease is most associated with ventricular tachycardia. • Therapy should be directed at the inciting cause with primary myocardial disease a likely complicating issue. Class I anti-arrhythmic medications (lidocaine, procainamide, quinidine, propafenone) are often effective in VT patients with clinical signs of cardiac insufficiency while at rest, if multimorphic VT is noted, R waves are superimposed onto T waves on ECG, or if the heart rate is excessive for the age group patient. • Although not as rapidly acting as lidocaine, magnesium sulfate (2–4 mg/kg q 2 minutes) can be useful to control ventricular tachycardia with a total dose not to exceed 50 mg/kg. • The use of corticosteroids may be indicated if primary cardiac disease is deemed to be the inciting cause. Valvular pathology is probably the most common cause of significant murmurs in the horse. These are sometimes accompanied by physiological disturbances of blood flow and exert consequent effects on the well-being and exercise capacity of the horse. In particular, defects of the mitral valve appear somewhat more likely to result in clinically significant murmurs. Mild, chronic, progressive valvular disease resulting in systolic murmurs form the commonest group of acquired disorders of the equine heart. The murmurs are usually of low intensity, and the sounds remain localized over the affected valve. Serious valvular dysfunction results in poor exercise tolerance and prolonged recovery after exercise. There is usually a high resting heart rate which shows a poor recovery rate to normal resting levels following even limited exercise but some cases affected by apparently severe murmurs show no detectable clinical effect on either performance or well-being. Causes of valvular regurgitation are listed in Table 4.1. Further discussion of more common causes is given below. Table 4.1 Causes of valvular regurgitation • Mitral regurgitation. The murmur of mitral insufficiency is holo- or pan-systolic, typically band-shaped and is loudest over the fifth intercostal space, radiating caudo-dorsally if it is severe. It may be an incidental finding or horses may show signs of poor performance or clinical signs of heart failure. The tolerance is often largely dependent on the type of work the horse is doing, with horses in vigorous training showing an expected poorer tolerance for the condition. In some horses the condition may be complicated by atrial fibrillation which further compromises cardiac function. Depending on the cause other clinical signs may be present such as fever, weight loss or polyarthritis which can occur in cases of bacterial endocarditis. • Aortic regurgitation. The murmur of aortic insufficiency is typically pan, holo or early diastolic decrescendo and has its point of maximal intensity over the aortic valve in the left fifth intercostal space and radiates variable distances ventrally. It may be musical or have a ‘creaking’ quality in some horses. In most horses, aortic regurgitation (AR) is an incidental finding and commonly occurs as a result of valvular degeneration in horses greater than 10 years of age. The existing murmur can vary greatly in intensity and is not indicative of the prognosis. Although degenerative valvular changes are generally regarded as being slowly progressive, studies have indicated that horses with AR have a shorter survival time and are more prone to developing congestive heart failure or sudden death. The quality of the arterial pulses is a good indicator of the severity of the AR as bounding pulses are associated with volume overload and moderate to severe AR with a poorer prognosis. • Tricuspid regurgitation. A soft, grade 2–3 pansystolic murmur with the point of maximal intensity on the right side of the tricuspid valve is most commonly found incidentally. Endocarditis less commonly affects the tricuspid valve and thus associated signs are not frequently seen. The prognosis for horses with TR is generally good. • Horses which are seen alive show an abrupt onset of severe respiratory distress and pulmonary edema, which is often audible and may be evident as a blood-stained, frothy, bilateral nasal discharge and cyanosis. • Echocardiography is diagnostic. • Treatment is supportive and dictated by the cardiovascular state although the prognosis is frequently poor. • Most horses with bacterial endocarditis present with intermittent pyrexia, weight loss, depression, malaise, intermittent lameness and clinical indications of cardiac insufficiency attributable to the chamber/side of heart affected. • A predisposing condition or concurrent infection or the history of such may be present but many horses present with no other obvious signs of disease and no history of a prior disorder. • Valvular lesions often incite regurgitant murmurs best heard over the valve involved. Therefore, atrioventricular valve endocarditis results in a systolic murmur whereas aortic or pulmonic valve endocarditis results in a diastolic murmur. Mural endocarditis and small vascular lesions may not produce heart murmurs, especially if the right side of the heart is affected. The quality or intensity of the murmur may change during subsequent examinations and arrythmias may also be present. • Right-sided vegetative lesions that dislodge may create embolic pneumonia or focal pulmonary abscesses, whereas left heart vegetations can dislodge to spread septic emboli to other organ systems with swelling of joints and tendon sheaths commonly seen. • Diagnostics include serial blood culture during or just after febrile episodes, CBC, chemistry, echocardiography and thoracic radiography. • Complete blood counts frequently demonstrate a moderate to marked neutrophilic leukocytosis and hyperfibrinogenemia. • Treatment is initially broad-spectrum antimicrobials with long-term (4–8 weeks) antimicrobial selection based on blood culture results. Bacteremia is considered continuous but the number of bacteria at any given time may fluctuate. • Clinical signs may be suggestive but echocardiography is fundamentally diagnostic where the thickened pericardium and the presence of variable amounts and consistency of effusion will usually be obvious. • Pericardiocentesis with fluid analysis and culture may be required to identify the causal agent and its susceptibility profile. Streptococcus spp., Actinobacillus equuli, Pseudomonas aeruginosa and Pasteurella sp. have all been reported. Pericardiocentesis may be performed between the fourth, fifth or sixth left intercostal space midway between the shoulder level and the sternum. This technique is relatively easy and safe. Simultaneous ultrasound may provide accurate guidance for the aspiration needle. Normal pericardial fluid is limited in volume and contains few cells and low protein levels, whereas in most cases of pericarditis, whether septic or not, both cell and protein content increase markedly. • The electrocardiogram of horses affected with pericarditis shows a characteristically diminished QRS voltage and/or repeated variations in QRS amplitude, which may be attributable to the movement of the heart within the distended pericardial sac. • Thoracic radiography may also be used to demonstrate an enlarged, rounded cardiac silhouette but is limited by any concurrent pleural fluid and the technical problems of thoracic radiography in all but the smaller horses. • CBC results are variable with the majority of horses demonstrating a moderate to marked neutrophilic leukocytosis with elevated fibrinogen. Animals suffering from pericardial tamponade will frequently present with azotemia due to decreased cardiac output and subsequent reduced renal output. Central venous pressure (if routinely monitored) greater than 10–12 mmHg is suggestive of cardiac tamponade and should prompt further diagnostics/therapy. • Post-mortem examination reveals a markedly thickened pericardial sac and the presence of exudate which may be purulent or partially organized and, in longstanding cases, fibrinous. There is commonly some intercurrent valvular endocarditis. • Therapy is aimed at the inciting cause with broad-spectrum antibiotics and anti-inflammatory medications initially; specific culture results may dictate longer-term treatment. • Pericardiocentesis is indicated in all cases of septic effusion and cases of cardiac tamponade. Electrocardiographic monitoring during the drainage procedure allows recognition of arrthymias. • Pericardial lavage can be performed in cases of septic pericarditis. It should be repeated daily until the accumulation of fluid declines (<1 L in 12 hours for an adult), clinical signs have improved and the cytological characteristics of the fluid indicate the presence of less inflammatory cells. • Surgery is rarely required but could be considered in cases of constrictive pericarditis that are non-responsive to other therapies. • Traditionally the prognosis for horses affected by pericarditis has been poor, however, there have been recent reports of successful outcomes.
Disorders of the cardiovascular system
Part 1: The heart and blood vessels
Developmental disorders
Persistent neonatal pulmonary hypertension
Diagnosis and treatment
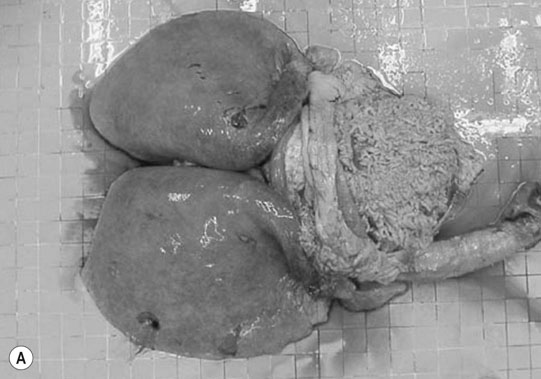
Patent ductus arteriosus (PDA) (Fig. 4.1)
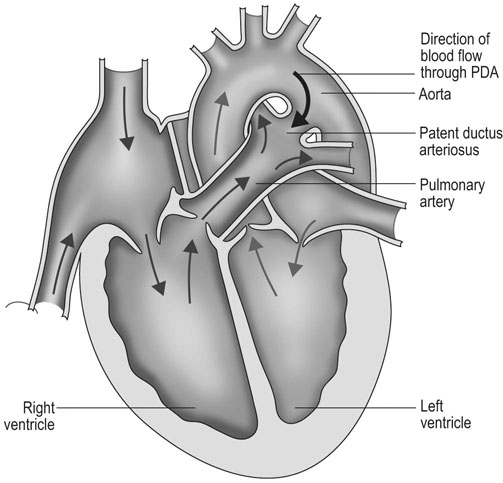
Diagnosis and treatment
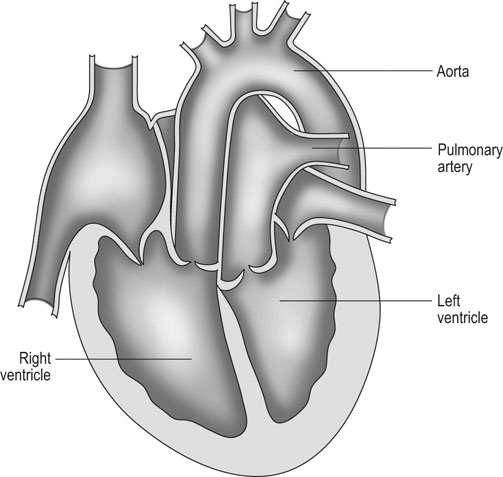
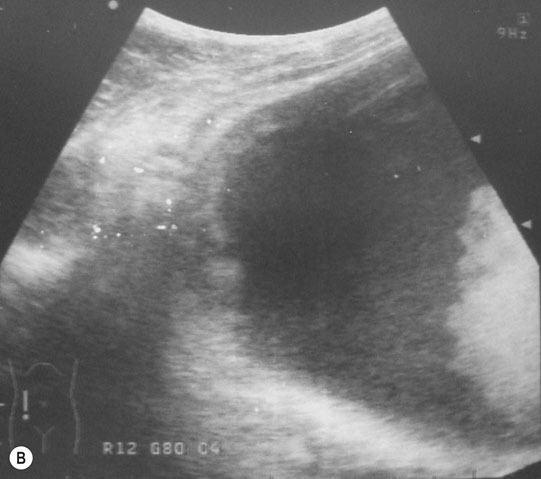
Ventricular septal defect (VSD) (Figs. 4.2–4.5)
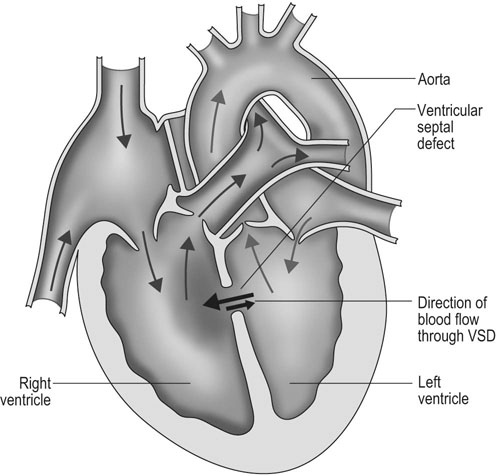
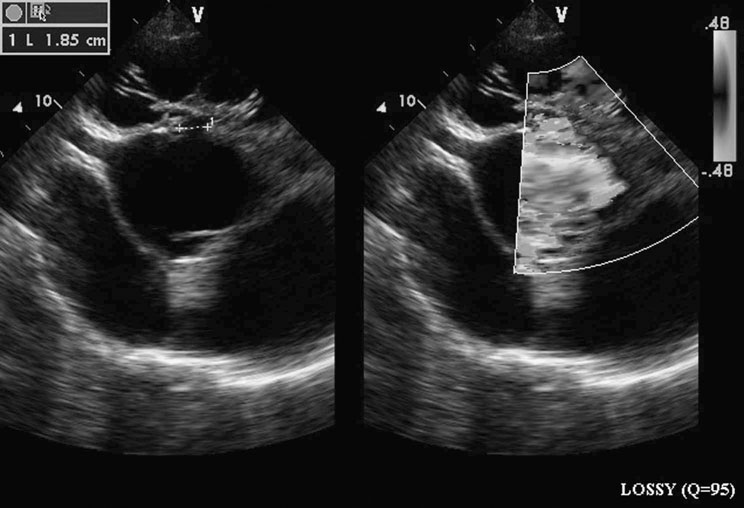
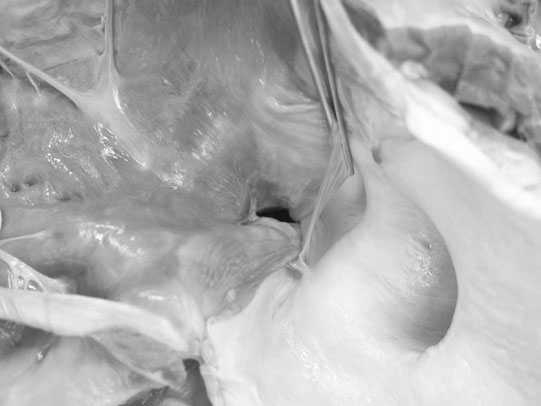
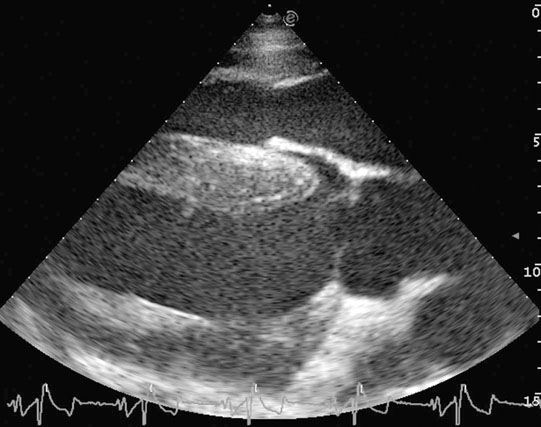
Diagnosis
Treatment and prognosis
Atrial septal defect (ASD) (Fig. 4.6)
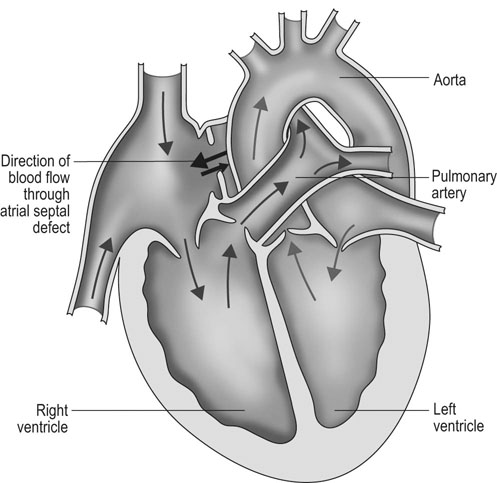
Diagnosis and treatment
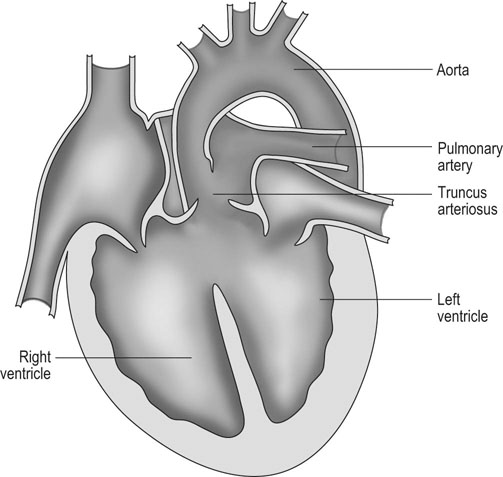
Tetralogy of Fallot (Figs. 4.7 & 4.8)
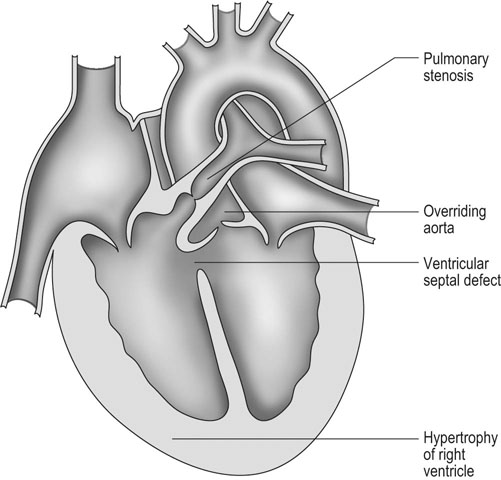
Diagnosis and treatment
Tricuspid atresia
Diagnosis and treatment
Non-infectious disorders
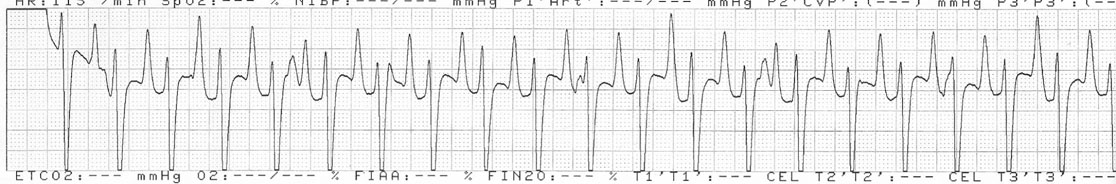
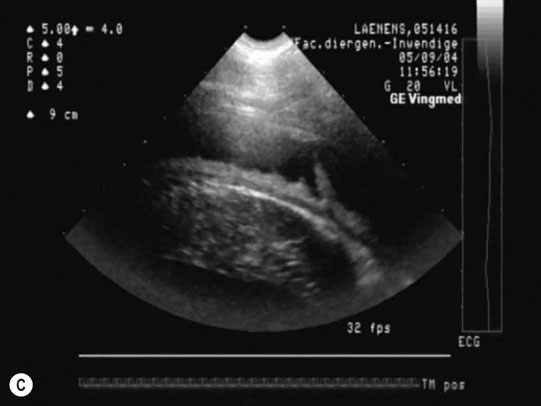
Dysrhythmias: atrial fibrillation (Fig. 4.11)
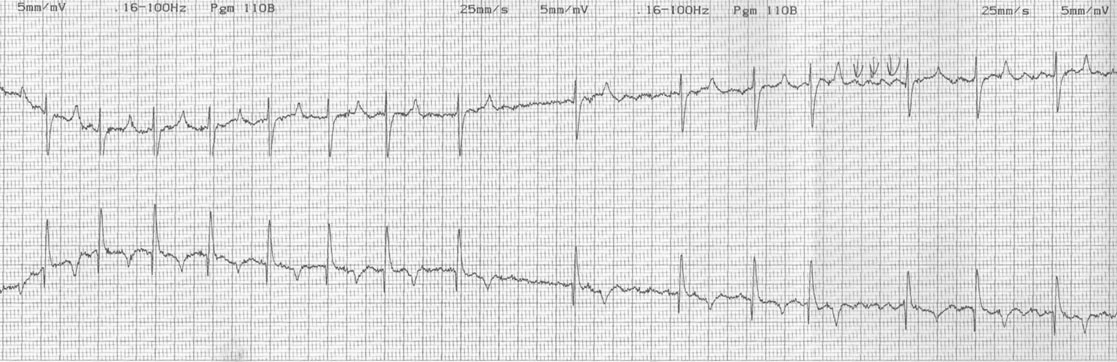
Diagnosis and treatment
Premature atrial beats (PAB) (Fig. 4.12)
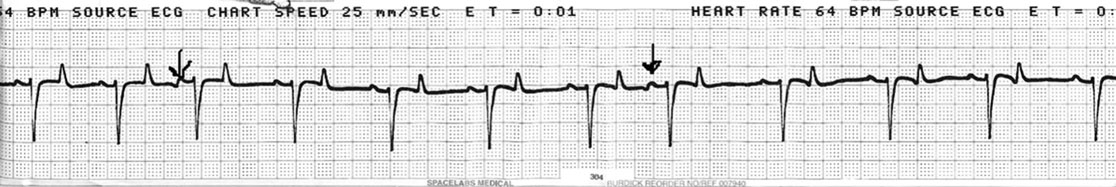
Diagnosis and treatment
Ventricular premature contractions (VPC) (Figs. 4.13 & 4.14)
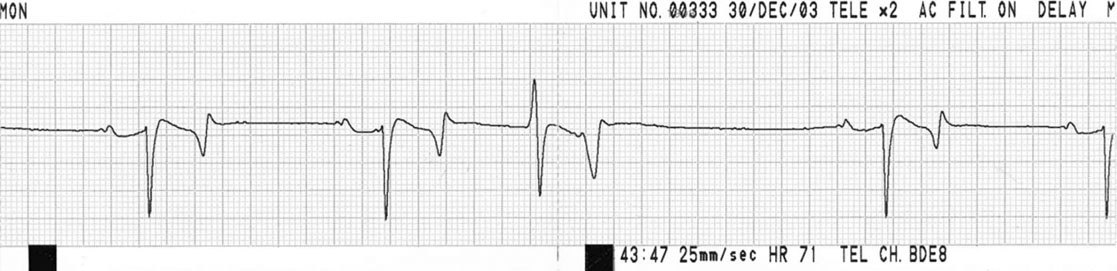
Diagnosis
Treatment
Acquired valvular heart disease
Mitral insufficiency
Aortic insufficiency
Tricuspid insufficiency
Degenerative thickening
Degenerative thickening
Degenerative thickening
Idiopathic disease
Idiopathic disease
Idiopathic disease
Prolapse of the valve
Prolapse of the valve
Prolapse of the valve
Ruptured chordae tendinae
Congenital valvular disease
Congenital valvular disease
Bacterial endocarditis
VSD
Bacterial endocarditis
Non-infective valvulitis
Non-infective valvulitis
Pulmonary hypertension
Primary or ischemic myocardial disease
Ruptured aortic sinus aneurysm
Myocardial disease
Congenital malformation of the valve
Ruptured chordae tendinae
Chronic tachycardia
Clinical signs
Ruptured chordae tendinae (Figs. 4.15 & 4.16)
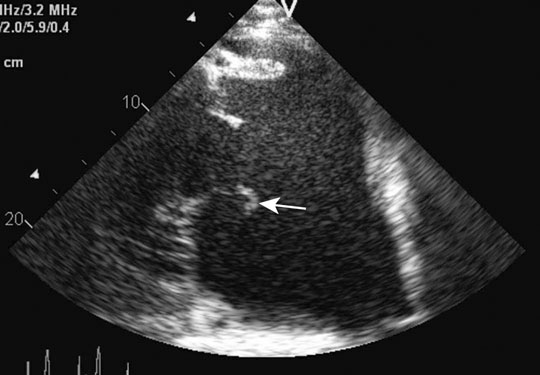
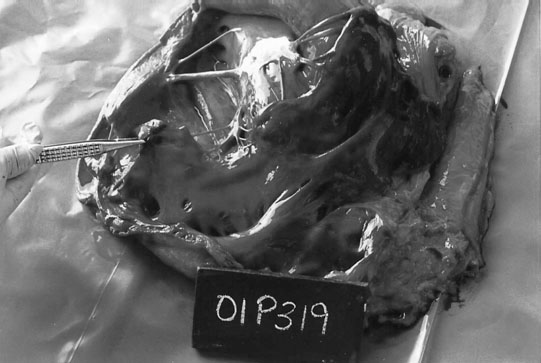
Clinical signs, diagnosis and treatment
Endocarditis (Figs. 4.17–4.24)
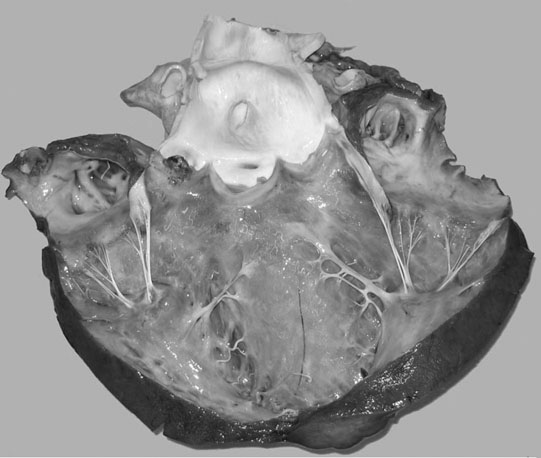
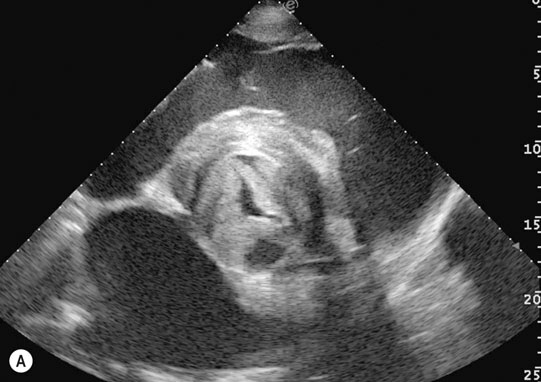
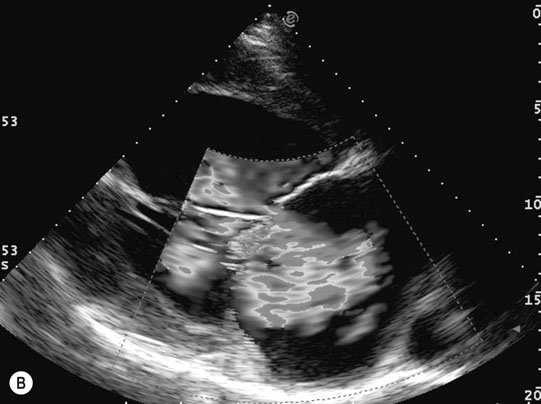
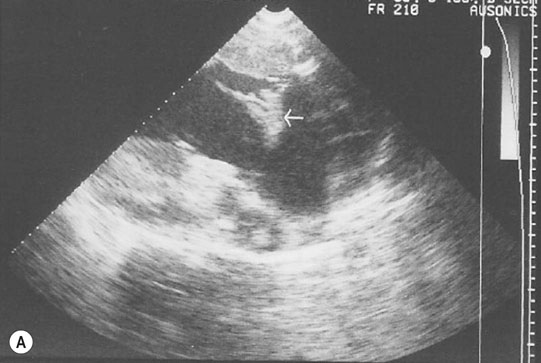
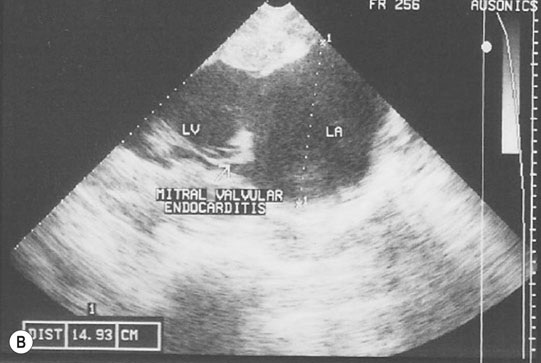
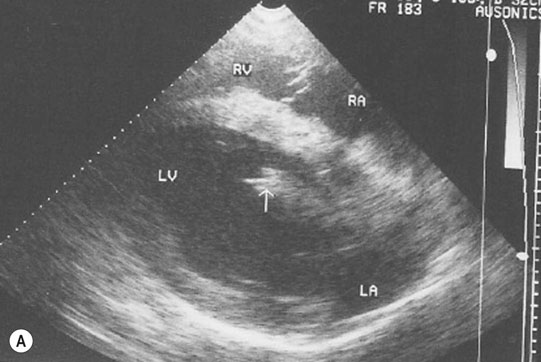
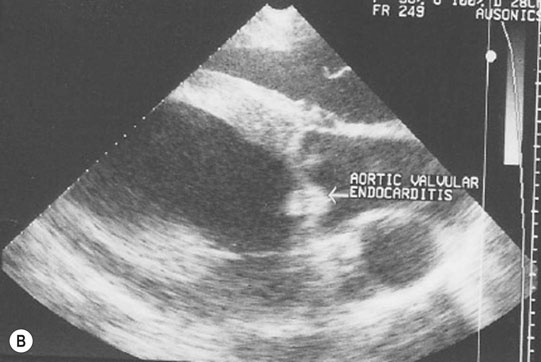
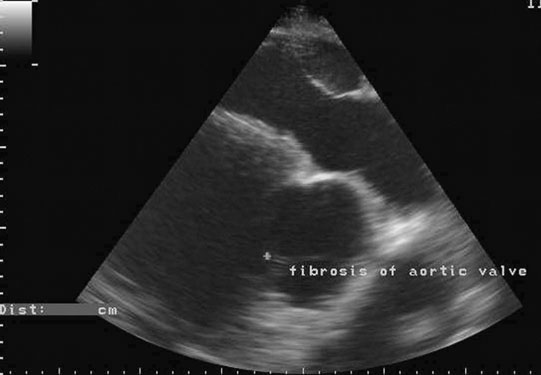


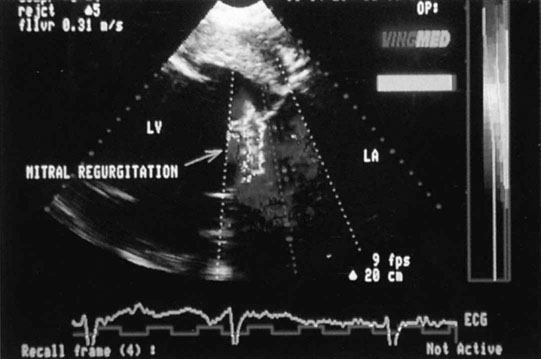
Clinical signs
Diagnosis and treatment
Pericarditis (Figs. 4.25–4.30)
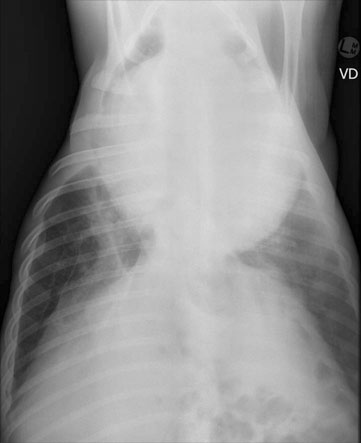


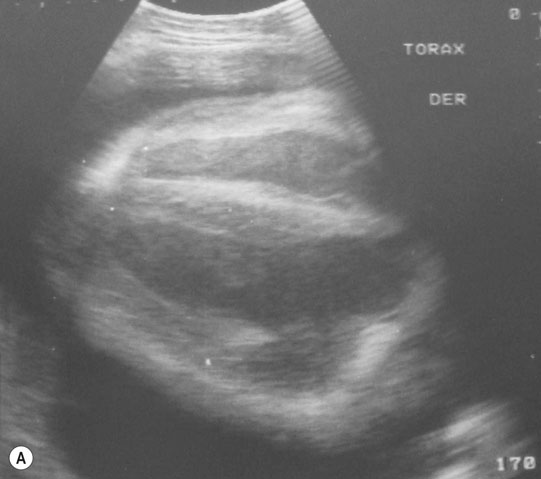

Diagnosis
Treatment and prognosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree